Summary
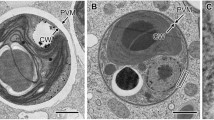
The photocytes and other endodermal cells composing the wall of the meridional canals of the comb-jelly, Mnemiopsis leidyi, were investigated by transmission electron microscopy. Although many of these cells possess distinctive features such as a ciliary apparatus, lysosome-like bodies or vacuoles, they share with photocytes the presence of a network of rough endoplasmic reticulum (RER) whose cisternae enwrap large mitochondria and are aligned along the subsurface of the plasma membrane. A stereological analysis of organelle content in photocytes confirms the prominence of the RER in these cells and a shift of RER from mitochondria to plasma membrane subsurface in photocytes induced to luminesce by the mitochondrial inhibitor dinitrophenol. Photocytes and other endodermal cells of the meridional canals are interconnected by numerous gap junctions which, among photocytes, often form symmetrical triads with cortical cisternae and mitochondria. The gap junctions and RER/mitochondria assemblages are interpreted as possible substrates for, respectively, conduction of luminescence excitation along the canals and for excitation-luminescence coupling. Neuntes occasionally make synapses with photocytes and other endodermal cells lying adjacent to the mesoglea.
Similar content being viewed by others
References
Anctil M (1985) Cholinergic and monoaminergic mechanisms associated with control of bioluminescence in the ctenophore Mnemiopsis leidyi. J Exp Biol (in press)
Anctil M, Shimomura O (1984) Mechanism of photoinactivation and reactivation in the bioluminescence system of the ctenophore Mnemiopsis. Biochem J 221:269–272
Anctil M, Descarries L, Watkins KC (1984) Distribution of [3H]noradrenaline and [3H]serotonin in photophores of Porichthys notatus. An electron-microscopic radioautographic analysis. Cell Tissue Res 235:129–136
Chang JJ (1954) Analysis of the luminescent response of the ctenophore, Mnemiopsis leidyi, to stimulation. J Cell Comp Physiol 44:365–394
Freeman G, Reynolds GT (1973) The development of bioluminescence in the ctenophore Mnemiopsis leidyi. Dev Biol 31:61–100
Hernandez-Nicaise M-L (1973) The nervous system of ctenophores. III. Ultrastructure of synapses. J Neurocytol 2:249–263
Labas YA (1977a) Triggering and regulatory mechanisms of the ciliary beating in ctenophora. I. Coordination of the ciliary beating with the intracellular bioluminescence and muscle contractions. Tsitologiya 19:514–521
Labas YA (1977b) Triggering and regulatory mechanisms of ciliary motion in the ctenophore Bolinopsis. II. Effect of ionic changes on ciliary apparatus and bioluminescence system of a ctenophore. Tsitologiya 19:644–654
Labas YA, Mashanskii VF (1976) Structural basis of luminescence in ctenophores. Biol Morya 1:57–66
Moore AR (1924) Luminescence in Mnemiopsis. J Gen Physiol 6:403–412
Morin JG (1974) Coelenterate bioluminescence. Muscatine L Lenhoff H (eds) Coelenterate biology: Reviews and new perspectives. Academic Press, New York, pp 397–438
Munn EA (1974) The structure of mitochondria. Academic Press, London, New York
Neu CW, Byers CR, Peek JM (1974) A technique for analysis of utilization-availability data. J Wildl Manage 38:541–545
Peters AW (1905) Phosphorescence in ctenophores. J Exp Zool 2:103–116
Satterlie RA, Case JF (1978) Gap junctions suggest epithelial conduction within the comb plates of the ctenophore Pleurobrachia bachei. Cell Tissue Res 193:87–91
Satterlie RA, Anderson PAV, Case JF (1980) Colonial coordination in anthozoans: Pennatulacea. Mar Behav Physiol 7:25–46
Ward WW, Seliger HH (1974a) Extraction and purification of calcium-activated photoproteins from ctenophores. Biochemistry 13:1491–1499
Ward WW, Seliger HH (1974b) Properties of mnemiopsin and berovin, calcium-activated photoproteins from the ctenophores Mnemiopsis sp. and Beroe ovata. Biochemistry 13:1500–1510
Ward WW, Seliger HH (1976) Action spectrum and quantum yield for the photoinactivation of mnemiopsin, a bioluminescent photoprotein from the ctenophore Mnemiopsis sp. Photochem. Photobiol. 23:351–363
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anctil, M. Ultrastructure of the luminescent system of the ctenophore Mnemiopsis leidyi . Cell Tissue Res. 242, 333–340 (1985). https://doi.org/10.1007/BF00214545
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00214545