Abstract
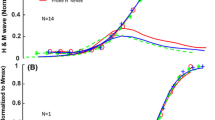
It was demonstrated that the soleus H-reflex was depressed for more than 10 s following a preceding passive dorsiflexion of the ankle joint. This depression was caused by activation of large-diameter afferents with receptors located in the leg muscles, as an ischaemic block of large-diameter fibres just below the knee joint abolished the depression, whereas a similar block just proximal to the ankle joint was ineffective. The depression of the H-reflex was not caused by changes in motoneuronal excitability, as motor-evoked potentials by magnetic brain stimulation were not depressed by the same passive dorsiflexion. Therefore it was concluded that the long-lasting depression is due to mechanisms acting at presynaptic level. The transmission of the monosynaptic Ia excitation from the femoral nerve to soleus motoneurones was not depressed by the ankle dorsiflexion. The depression thus seems to be confined to those afferents that were activated by the conditioning dorsiflexion. In parallel experiments on decerebrate cats, more invasive methods have complemented the indirect techniques used in the experiments on human subjects. A similar long-lasting depression of triceps surae monosynaptic reflexes was evoked by a preceding conditioning stimulation of the triceps surae Ia afferents. This depression was accompanied by a reduction of the monosynaptic Ia excitatory postsynaptic potential recorded intracellularly in triceps surae motoneurones, but not by changes in the input resistance or membrane potential in the motoneurones. Stimulation of separate branches within the triceps surae nerve demonstrated that the depression is confined to those afferents that were activated by the conditioning stimulus. This long-lasting depression was not accompanied by a dorsal root potential. It is concluded that the long-lasting depression is probably caused by a presynaptic effect, but different from the “classical” GABAergic presynaptic inhibition which is widely distributed among afferent fibres and accompanied by dorsal root potentials. It is more probably related to the phenomenon of a reduced transmitter release from previously activated fibres, i.e. a homosynaptic post-activation depression. The consequences of this post-activation depression for the interpretation of results on spinal mechanisms during voluntary movements in man are discussed.
Similar content being viewed by others
References
Ballegaard M, Hultborn H, Illert M, Nielsen J, Paul A, Wiese H (1991) Stretches of a muscle depress the transmission of its monosynaptic reflex (abstract). Eur J Neurosci [Suppl] 4: 298
Burke RE, Rudomin P (1977) Spinal neurons and synapses. In: Kandel ER (ed) Cellular biology of neurons. (Handbook of physiology, sect 1, The nervous system, vol I, part 2) Am Physiol Soc, Bethesda, pp 877–944
Crone C, Nielsen J (1989a) Spinal mechanisms in man contributing to reciprocal inhibition during voluntary dorsiflexion of the foot. J Physiol (Lond) 416:255–272
Crone C, Nielsen J (1989b) Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res 78:28–32
Crone C, Hultborn H, Jespersen B, Nielsen J (1987) Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol (Lond) 389:163–185
Crone C, Hultborn H, Mazières L, Morin C, Nielsen J, Pierrot Deseilligny E (1990) Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81:35–45
Curtis DR, Eccles JC (1960) Synaptic action during and after repetitive stimulation. J Physiol (Lond) 150:374–398
Davis GW, Murphey RK (1994) Long-term regulation of shortterm transmitter release properties: retrograde signaling and synaptic development. Trends Neurosci 17:9–13
Eccles JC (1964) The physiology of synapses. Springer, Berlin Heidelberg New York, p 316
Gerilovsky L, Tsvetinov P, Trenkova G (1989) Peripheral effects on the amplitude of monopolar and bipolar H-reflex potentials from the soleus muscle. Exp Brain Res 76:173–181
Hirst GD, Redman SJ, Wong K (1981) Post-tetanic potentiation and facilitation of synaptic potentials evoked in cat spinal motoneurones. J Physiol (Lond) 321:97–109
Honig MG, Collins WF, Mendell LM (1983) α-Motoneuron EPSPs exhibit different frequency sensitivities to single Ia-afferent fiber stimulation. J Neurophysiol 49:886–901
Hultborn H, Pierrot-Deseilligny E, Wigström H (1979) Recurrent inhibition and afterhyperpolarization following motoneuronal discharge in the cat. J Physiol (Lond) 297:253–266
Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E (1987) Assessing changes in presynaptic inhibition of Ia fibres: a study in man and the cat. J Physiol (Lond) 389:729–756
Katz R, Morin C, Pierrot-Deseilligny E, Hibino R (1977) Conditioning of H reflex by a preceding subthreshold tendon reflex stimulus. J Neurol Neurosurg Psychiatry 40:575–580
Koerber HR, Mendell LM (1991) Modulation of synaptic transmission at Ia-afferent connections on motoneurons during high-frequency afferent stimulation: dependence on motor task. J Neurophysiol 65:1313–1320
Kuno M (1964) Mechanism of facilitation and depression of the excitatory synaptic potential in spinal motoneurones. J Physiol (Lond) 175:100–112
Lüscher H-R, Ruenzel P, Henneman E (1983) Effects of impulse frequency, PTP, and temperature on responses elicited in large populations of motoneurons by impulses in single la-fibers. J Neurophysiol 50:1045–1058
Magladery JW, Teasdall RD, Park AM, Languth HW (1952) Electrophysiological studies of reflex activity in patients with lesions of the nervous system. I. A comparison of spinal motoneruone excitability following afferent nerve volleys in normal person and patients with upper motor neurone lesions. Bull Johns Hopkins Hosp 91:219–244
Mark RF, Coquery JM, Paillard J (1968) Autogenic reflex effects of slow or steady stretch of the calf. Exp Brain Res 6:130–145
Martin AR (1977) Junctional transmission. II. Presynaptic mechanisms. In: Kandel E (ed) Cellular biology of neurons. (Handbook of physiology, sect 1, The nervous system, vol I) Am Physiol Soc, Bethesda, pp 329–355
Meinck HM (1980) Facilitation and inhibition of the human H-reflex as a function of the amplitude of the control reflex. Electroencephalogr Clin Neurophysiol 48:203–211
Mendell LM (1984) Modifiability of spinal synapses. Physiol Rev 64:260–324
Nielsen J, Hultborn H (1993) Regulated properties of motoneurons and primary afferents: new aspects on possible spinal mechanisms underlying spasticity. In: Thilmann A, Burke D, Rymer Z (eds) Spasticity: mechanisms and management. Springer, Berlin Heidelberg New York, pp 177–191
Nielsen J, Kagamihara Y, Crone C, Hultborn H (1992) Central facilitation of Ia inhibition during tonic ankle dorsiflexion revealed after blockade of peripheral feedback. Exp Brain Res 88:651–656
Nielsen J, Petersen N, Ballegaard M, Biering-Sørensen F, Kiehn O (1993) H-reflexes are less depressed following muscle stretch in spastic spinal cord injured patients than in healthy subjects. Exp Brain Res 97:173–176
Nielsen J, Crone C, Sinkjær T, Toft E, Hultborn H (1995a) Central control of reciprocal inhibition during fictive dorsiflexion in man. Exp Brain Res 104:99–106
Nielsen J, Petersen N, Crone C (1995b) Changes in transmission across synapses of Ia afferents in spastic patients. Brain 118:995–1004
Paillard J (1955) Reflexes et regulation d'origine proprioceptive chez l'homme. Thesis, Libraire Arnette, Paris, pp 1–293
Pollock IJ, Davis L (1930) The reflex activities of a decerebrate animal. J Comp Neurol 50:377–411
Prochazka A, Trend P, Hulliger M, Vincent S (1989) Ensemble proprioceptive activity in the cat step cycle: towards a representative look-up chart. Prog Brain Res 80:61–74
Romanò C, Schieppati M (1987) Reflex excitability of human soleus motoneurones during voluntary shortening or lengthening contractions. J Physiol (Lond) 390:271–284
Rossi A, Mazzocchio R, Schieppati M (1988) The H reflex recovery curve reinvestigated: low-intensity conditioning stimulation and nerve compression disclose differential effects of presumed group Ia fibres in man. Hum Neurobiol 6:281–288
Rothwell JC, Day BL, Marsden CD (1986) Habituation and conditioning of the human long latency stretch reflex. Exp Brain Res 63:197–204
Schieppati M, Crenna P (1984) From activity to rest: gating of excitatory autogenetic afferences from the relaxing muscle in man. Exp Brain Res 56:448–457
Schieppati M, Poloni M, Nardone A (1985) Voluntary muscle release is not accompanied by H-reflex inhibition in patients with upper motor neuron lesions. Neurosci Lett 61:177–181
Schieppati M, Nardone A, Poloni M (1987) Changes in the normal pattern of H-reflex inhibition during muscle release in ALS. Adv Exp Med Biol 209:155–158
Táboříková H, Sax DS (1969) Conditioning of H-reflexes by a preceding subthreshold H-reflex stimulus. Brain 92:200–212
Zander Olsen P, Diamantopoulos E (1967) Excitability of spinal motor neurones in normal subjects and patients with spasticity, parkinsonian rigidity, and cerebellar hypotonia. J Neurol Neurosurg Psychiatry 30: 325–331
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hultborn, H., Illert, M., Nielsen, J. et al. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res 108, 450–462 (1996). https://doi.org/10.1007/BF00227268
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227268