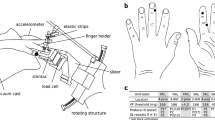
Summary
Impulses in single tactile units innervating the human glabrous skin were recorded percutaneously from the median nerve using tungsten electrodes. The units were classified as belonging to one of the four categories: fast adapting with small receptive fields (FA I), fast adapting with large receptive fields (FA II), slowly adapting with small fields (SA I), and slowly adapting with large fields (SA II). A small test object was lifted, positioned in space and replaced using the precision grip between fingers and thumb. The grip force, the load force (vertical lifting force), the vertical movements of the object and vibrations (accelerations) in the object were recorded. After being virtually silent between lifts, the FA I units whose fields contacted the object became highly active during the initial period of grip force increase (initial response). This was also true for most SA I units. Accordingly, most of the skin deformation changes took place at low grip forces (below ca. 1 N). Later, while the load and grip forces increased in parallel during isometric conditions, the FA I and SA I units continued firing but generally at declining impulse rates. As long as the object was held in the air, the SA I units generally maintained firing with a tendency to adaptation. A minority of the FA I unit also discharged, especially during periods of pronounced physiological muscle tremor. The SA I units usually became silent when the grip and load forces in parallel declined to zero during isometric conditions after the object had contacted the table. However, during the very release of the grip the FA I units and some SA I units showed brief burst discharges (release response). The FA II units responded distinctly to the mechanical transients associated with the start of the vertical movement and especially with the sudden cessation of movement at the terminal table contact. FA II units whose end organs were remotely located in relation to the skin areas in contact with the object also responded. Most FA II units also discharged at the initial touch and at the release of the object, albeit less reliably than the type I units. In addition to weak dynamic responses during the phase of isometric force increase, the SA II units showed comparatively strong tonic responses while the object was held during static conditions. High firing rates also were maintained during long-lasting lifts. Moreover, it was established that the signals in SA II afferents were related to the three dimensional force profile in the grip. The results are discussed with regard to the possible implications for the control of precise manipulative movements.
Similar content being viewed by others
References
Angel RW, Eppler W, Iannone A (1965) Silent period produced by unloading of muscles during voluntary contractions. J Physiol (Lond) 180: 864–870
Burgess PR, Perl ER (1973) Cutaneous mechanoreceptors and nociceptors. In: Iggo A (ed) Somatosensory system. Handbook of sensory physiology, Vol II. Springer, Berlin, pp 29–78
Chambers MR, Andres KH, v Duering M, Iggo A (1972) The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J exp Physiol 57: 417–445
Darton K, Lippold OCJ, Shahani M, Shahani U (1985) Longlatency spinal reflexes in humans. J Neurophysiol 53: 1604–1618
Datta AK, Stephens JA (1981) The effect of digital nerve stimulation on the firing of motor units in human first dorsal interosseous muscle. J Physiol (Lond) 318: 501–510
Day BL, Marsden CD (1982) Accurate repositioning of the human thumb against unpredictable loads is dependent upon peripheral feed-back. J Physiol (Lond) 327: 393–407
Denny-Brown D (1966) The cerebral control of movement. The Liverpool University Press, Liverpool
Franke EK (1951) Mechanical impedance of the surface of the human body. J Appl Physiol 3: 582–590
Gandevia SC, McCloskey DI (1977a) Effects of related sensory inputs on motor performance in man studied through changes in perceived heaviness. J Physiol (Lond) 272: 653–672
Gandevia SC, McCloskey DI (1977b) Changes in motor commands, as shown by changes in perceived heaviness, during partial curarization and peripheral anaesthesia in man. J Physiol (Lond) 272: 673–689
Garnett R, Stephens JA (1980) The reflex responses of single motor units in human first dorsal interosseous muscle following cutaneous afferent stimulation. J Physiol (Lond) 303: 351–364
Garnett R, Stephens JA (1981) Changes in the recruitment threshold of motor units produced by cutaneous stimulation in man. J Physiol (Lond) 311: 463–473
Hulliger M, Nordh E, Thelin AE, Vallbo ÅB (1979) The responses of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. J Physiol (Lond) 291: 233–249
Iggo A (1974) Cutaneous receptors. In: Hubbard JI (ed) Peripheral nervous system. Plenum Press, New York, pp 347–404
Johansson RS (1978) Tactile sensibility in the human hand: receptive field characteristics of mechanoreceptive units in the glabrous skin area. J Physiol (Lond) 281: 101–123
Johansson RS, Landström U, Lundström R (1982a). Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res 244: 17–25
Johansson RS, Landström U, Lundström R (1982b). Sensitivity to edges of mechanoreceptive afferent units innervating the glabrous skin of the human hand. Brain Res 244: 27–32
Johansson RS, Vallbo ÅB (1979a) Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in the glabrous skin. J Physiol (Lond) 286: 283–300
Johansson RS, Vallbo ÅB (1979b) Detection of tactile stimuli. Thresholds of afferent units related to psychophysical thresholds in the human hand. J Physiol (Lond) 297: 405–422
Johansson RS, Vallbo ÅB (1980) Spatial properties of the population of mechanoreceptive units in the glabrous skin of the human hand. Brain Res 184: 353–366
Johansson RS, Vallbo ÅB (1983) Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci 6: 27–31
Johansson RS, Vallbo ÅB, Westling G (1980) Thresholds of mechanosensitive afferents in the human hand as measured with von Frey hairs. Brain Res 184: 343–351
Johansson RS, Westling G (1984a) Influences of cutaneous sensory input on the motor coordination during precision manipulation. In: von Euler C, Franzen O, Lindblom U, Ottoson D (eds) Somatosensory mechanisms. Macmillan Press, London, pp 249–260
Johansson RS, Westling G (1984b) Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res 56: 550–564
Johansson RS, Westling G (1987) Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Exp Brain Res 66: 141–154
Johnson KO (1983) Neural mechanisms of tactual form and texture discrimination. Fed Proc 42: 2542–2527
Kanda K, Desmedt JE (1983) Cutaneous facilitation of large motor units and motor control of human fingers in precision grip. In: Desmedt JE (ed) Motor control mechanisms in health and disease. Raven, New York, pp 253–261
Knibestöl M, Vallbo ÅB (1970) Single unit analysis of mechanoreceptor activity from the human glabrous skin. Acta Physiol Scand 80: 178–195
Knibestöl M (1975) Stimulus-response functions of slowly adapting mechanoreceptors in the human glabrous skin area. J Physiol (Lond) 245: 63–80
Loo CKC, McCloskey DI (1985) Effects of prior instruction and anaesthesia on long-latency responses to stretch in the long flexor of the human thumb. J Physiol (Lond) 365: 285–296
Marsden CD, Merton PA, Morton HB (1976) Servo action in the human thumb. J Physiol (Lond) 257: 1–44
Marsden CD, Merton PA, Morton HB (1977) The sensory mechanism of servoaction in human muscle. J Physiol (Lond) 265: 521–535
Matthews PBC (1984) Evidence from the use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol (Lond) 348: 383–415
McCloskey DI (1974) Muscular and cutaneous mechanisms in the estimation of the weights of grasped objects. Neuropsychologia 12: 513–520
McCloskey DI, Gandevia S (1978) Role of inputs from skin, joints and muscles and corollary discharges, in human discriminatory tasks. In: Gordon G (ed) Active touch. Pergamon, Oxford, pp 177–187
McCloskey DI, Gandevia S, Potter ED, Colebatch SG (1983) Muscle sense and effort: motor commands and judgements about muscular contractions. In: Desmedt JE (ed) Motor control mechanisms in health and disease. Raven Press, New York, pp 151–168
Moberg E (1962) Criticism and study of methods for examining sensibility in the hand. Neurology 12: 8–19
Mott FW, Sherrington CS (1895) Experiments upon the influence of sensory nerves upon movement and nutrition of the limbs. Proc R Soc B 57: 481–488
Ochoa J, Torebjörk E (1983) Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervation the human hand. J Physiol (Lond) 342: 633–654
Petit J, Galifret Y (1978) Sensory coupling function and mechanical properties of the skin. In: Gordon G (ed) Active touch. Pergamon, Oxford, pp 19–27
Phillips JR, Johnson KO (1981a) Tactile spatial resolution. II. Neural representation of bars, edges, and gratings in monkey primary afferents. J Neurophysiol 46: 1192–1203
Phillips JR, Johnson KO (1981b) Tactile spatial resolution. III. A continuum mechanics model of skin predicting mechanoreceptor responses to bars, edges, and gratings. J Neurophysiol 46: 1204–1225
Pubols B, Pubols L (1983) Tactile receptor discharge and mechanical properties of the glabrous skin. Fed Proc 42: 2528–2535
Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB (1968) The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol 31: 301–334
Torebjörk HE, Hagbarth KE, Eklund G (1978) Tonic finger flexion reflex induced by vibratory activation of digital mechanoreceptors. In: Gordon G (ed) Active touch. Pergamon, Oxford, pp 197–203
Twitchell TE (1954) Sensory factors in purposive movement. J Neurophysiol 17: 239–252
Vallbo ÅB, Hagbarth KE (1968) Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol 21: 270–289
Vallbo Å, Johansson RS (1984) Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Human Neurobiol 3: 3–14
Vallbo ÅB, Olsson KÅ, Westberg K-G, Clark FJ (1984) Microstimulation of single tactile afferents from the human hand. Brain 107: 727–749
Verrillo RT (1965) Temporal summation in vibrotactile sensitivity. J Acoust Soc Am 37: 843–846
Westling G, Johansson RS (1984) Factors influencing the force control during precision grip. Exp Brain Res 53: 277–284
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Westling, G., Johansson, R.S. Responses in glabrous skin mechanoreceptors during precision grip in humans. Exp Brain Res 66, 128–140 (1987). https://doi.org/10.1007/BF00236209
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00236209