Summary
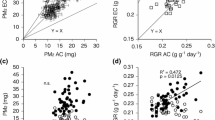
Which factors cause fast-growing plant species to achieve a higher relative growth rate than slow-growing ones? To answer this question 24 wild species were grown from seed in a growth chamber under conditions of optimal nutrient supply and a growth analysis was carried out. Mean relative growth rate, corrected for possible ontogenetic drift, ranged from 113 to 356 mg g−1 day−1. Net assimilation rate, the increase in plant dry weight per unit leaf area and unit time, varied two-fold between species but no correlation with relative growth rate was found. The correlation between leaf area ratio, the ratio between total leaf area and total plant weight, and relative growth rate was very high. This positive correlation was mainly due to the specific leaf area, the ratio between leaf area and leaf weight, and to a lesser extent caused by the leaf weight ratio, the fraction of plant biomass allocated to the leaves. Differences in relative growth rate under conditions of optimum nutrient supply were correlated with the soil fertility in the natural habitat of these species. It is postulated that natural selection in a nutrient-rich environment has favoured species with a high specific leaf area and a high leaf weight ratio, and consequently a high leaf area ratio, whereas selection in nutrient-poor habitats has led to species with an inherently low specific leaf area and a higher fraction of root mass, and thus a low leaf area ratio.
Similar content being viewed by others
References
Aerts R (1989) Nitrogen-use-efficiency in relation to nitrogen availability and plant community composition. In: Lambers H, Cambridge ML, Konings H, Pons TL (eds) Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants, SPB Academic Publishing, The Hague, pp 285–297
Berendse F, Aerts R (1987) Nitrogen-use-efficiency: a biologically meaningful definition? Funct Ecol 1:293–296
Boot RGA (1987) The significance of size and morphology of root systems for nutrient acquisition and competition. In: Lambers H, Cambridge ML, Konings H, Pons TL (eds) Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants, SPB Academic Publishing, The Hague, pp 299–311
Brouwer R (1963) Some aspects of the equilibrium between overground and underground plant parts. Meded Inst Biol Scheik Onderz Landb Gewas 213:31–39
Chapin FS (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Corré WJ (1983) Growth and morphogenesis of sun and shade plants. III. The combined effects of light intensity and nutrient supply. Acta Bot Neerl 32:277–294
Dijkstra P (1989) Cause and effect of differences in specific leaf area. In: Lambers H, Cambridge ML, Konings H, Pons TL (eds) Causes and Consequences of Variation in Growth Rate and Productivity in Plants, SPB Academic Publishing, The Hague, pp 125–140
Dijkstra P, Lambers H (1986) Photosynthesis and respiration of two inbred lines of Plantago major L. differing in relative growth rate. In: Marcelle R, Clijsters H, Van Poucke M (eds) Biological Control of Photosynthesis, Martinus Nijhoff Publishers, The Hague, pp 251–255
Eagles CF (1967) The effect of temperature on vegetative growth in climatic races of Dactylis glomerata in controlled environments. Ann Bot 31:31–39
Elias CO, Chadwick MJ (1979) Growth characteristics of grass and legume cultivars and their potential for land reclamation. J Appl Ecol 16:537–544
Ellenberg HE (1979) Zeigerwerte der Gefässpflanze Mitteleuropas. Scripta Geobotanica 9
Evans GC (1972) The Quantitative Analysis of Plant Growth. Blackwell Scientific Publications, Oxford
Grime JP (1979) Plant Strategies & Vegetation processes. John Wiley & Sons, Chichester
Grime JP, Hunt R (1975) Relative growth-rate: its range and adaptive significance in a local flora. J Ecol 63:393–422
Higgs DEB, James DB (1969) Comparative studies on the biology of upland grasses. I. Rate of dry matter production and its control in four grass species. J Ecol 57:553–563
Hunt R, Nicholls AO, Fathy SA (1987) Growth and root-shoot partitioning in eighteen British grasses. Oikos 50:53–59
Jarvis PG, Jarvis MS (1964) Growth rates of woody plants. Physiol Plant 17:654–666
Joyner SP (1985) SAS/STAT Guide for Personal Computers, Version 6 Edition. SAS Institute Inc, Cary, NC. pp 269–336
Körner C, Renhardt U (1987) Dry matter partitioning and root length/leaf area rations in herbaceous perennial plants with diverse altitude distribution. Oecologia 74:411–418
Konings H (1989) Physiological and morphological differences between plants with a high NAR or a high LAR as related to environmental conditions. In: Lambers H, Cambridge ML, Konings H, Pons TL (eds) Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants, SPB Academic Publishing, The Hague, pp 101–123
Konings H, Koot E, Tijman-De Wolf A (1989) Growth characteristics, nutrient allocation and photosynthesis of Carex species from floating fens. Oecologia 80:111–121
Lambers H, Dijkstra P (1987) A physiological analysis of genotypic variation in relative growth rate: Can growth rate confer ecological advantage? In: Van Andel J, Bakker JP, Snaydon RW (eds) Disturbance in Grasslands, Junk Publishers, Dordrecht, pp 237–252
Merino J, Field C, Mooney HA (1984) Construction and maintenance costs of mediterranean-climate evergreen and deciduous leaves. II. Biochemical pathway analysis. Acta Oecol/Oecol Plant 5:211–229
Monk CD (1966) An ecological significance of evergreens. Ecology 47:504–505
Pons TL (1977) An ecophysiological study in the field layer of ash coppice. II. Experiments with Geum urbanum and Cirsium palustre in different light intensities. Acta Bot Neerl 26:251–263
Poorter H (1989a) Growth analysis: towards a synthesis of the classical and the functional approach. Physiol Plant 75:237–244
Poorter H (1989b) Interspecific differences in relative growth rate: On ecological causes and physiological consequences. In: Lambers H, Cambridge ML, Konings H, Pons TL (eds) Causes and Consequences of Variation in Growth Rate and Productivity in Plants, SPB Academic Publishing, The Hague, pp 45–68
Poorter H, Lewis C (1986) Testing differences in PGR: a method avoiding curve fitting and pairing. Physiol Plant 67:223–226
Poorter H, Pot CS, Lambers H (1988) The effect of an elevated CO2 concentration on growth, photosynthesis and respiration of Plantago major, a rosette plant. Physiol Plant 73:553–559
Potter JR, Jones JW (1977) Leaf area partitioning as an important factor in growth. Plant Physiol 59:10–14
Roetman E, Sterk AA (1986) Growth of microspecies of different sections of Taraxacum in climatic chambers. Acta Bot Neerl 35:5–22
Small E (1972) Photosynthetic rates in relation to nitrogen recycling as an adaptation to nutrient deficiency in peat bog plants. Can J Bot 50:2227–2233
Smeets L, Garretsen F (1986) Growth analyses of tomato genotypes grown under low night temperatures and low light intensity. Euphytica 35:701–715
Tilman GD (1984) Plant dominance along an experimental nutrient gradient. Ecology 65:1445–1453
Van der Meijden R, Weeda EJ, Adema FACB, De Joncheere GJ (1987) Flora van Nederland. Wolters-Noordhoff, Groningen
Van Dijk H, Van Delden W (1981) Genetic variability in Plantago species in relation to their ecology. Part 1: Genetic analysis of the allozyme variation in P. major subspecies. Theor Appl Genet 60:285–290
Wilson D (1982) Response to selection for dark respiration rate of mature leaves in Lolium perenne and its effects on growth of young plants and simulated swards. Ann Bot 49:303–312
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Poorter, H., Remkes, C. Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83, 553–559 (1990). https://doi.org/10.1007/BF00317209
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317209