Abstract
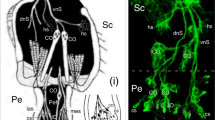
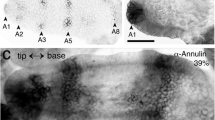
We have used electron-microscopic studies, bromodeoxyuridine (BrdU) incorporation and antibody labeling to characterize the development of the Drosophila larval photoreceptor (or Bolwig's) organ and the optic lobe, and have investigated the role of Notch in the development of both. The optic lobe and Bolwig's organ develop by invagination from the posterior procephalic region. After cells in this region undergo four postblastoderm divisions, a total of approximately 85 cells invaginate. The optic lobe invagination loses contact with the outer surface of the embryo and forms an epithelial vesicle attached to the brain. Bolwig's organ arises from the ventralmost portion of the optic lobe invagination, but does not become incorporated in the optic lobe; instead, its 12 cells remain in the head epidermis until late in embryogenesis when they move in conjunction with head involution to reach their final position alongside the pharynx. Early, before head involution, the cells of Bolwig's organ form a superficial group of 7 cells arranged in a ‘rosette’ pattern and a deep group of 5 cells. Later, all neurons move out of the surface epithelium. Unlike adult photoreceptors, they do not form rhabdomeres; instead, they produce multiple, branched processes, which presumably carry the photopigment. Notch is essential for two aspects of the early development of the visual system. First, it delimits the number of cells incorporated into Bolwig's organ. Second, it is required for the maintenance of the epithelial character of the optic lobe cells during and after its invagination.
Similar content being viewed by others
References
Artavanis-Tsakonas S (1988) The molecular biology of the Notch locus and the fine tuning of differentiation in Drosophila. Trends Genet 4:95–100
Bier E, Vaessin H, Shepherd S, Lee K, McCall K, Barbel S, Ackerman L, Carretto R, Uemura T, Grell E, Jan LY, Jan YN (1989) Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev 3:1273–1287
Bodmer R, Carretto R, Jan YN (1989) Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron 3:21–32
Bolwig N (1946) Senses and sense organs of the anterior end of the house fly larvae. Vidensk Med Dansk Naturh Foren 109:81–217
Cagan RL, Ready DF (1989) Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev 3:1099–1112
Campos-Ortega JA (1988) Cellular interactions during early neurogenesis of Drosophila melanogaster. Trends Neurosci 11:400–405
Campos-Ortega JA, Hartenstein V (1985a) Development of the nervous system. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry, and pharmacology, vol 5. Pergamon Press, Oxford, pp 49–85
Campos-Ortega JA, Hartenstein V (1985b) The embryonic development of Drosophila melanogaster. Springer, Berlin
Chen TY (1929) On the development of imaginal buds in normal and mutant Drosophila melanogaster. J Morphol 47:135–199
Copenhaver PF, Taghert PH (1990) Neurogenesis in the insect enteric nervous system: generation of premigratory neurons from an epithelial placode. Development 109:17–28
Corbin V, Michelson AM, Abmayr SM, Neel B, Alcamo E, Maniatis T, Young MW (1991) A role for the Drosophila neurogenic genes in mesoderm differentiation. Cell 67:311–323
Ellsworth JK (1933) The photoreceptive organs of fleshfly larva, Lucilia sericata (Meigen): an experimental and anatomical study. Ann Entomol Soc Am 26:200–215
Fehon RG, Johansen K, Rebay I, Artavanis-Tsakonas S (1991) Complex cellular and subcellular regulation of Notch expression during embryonic and imaginal development of Drosophila: implications for Notch function. J Cell Biol 113:657–669
Hartenstein V (1988) Development of Drosophila larval sensory organs: spatiotemporal pattern of sensory neurones, peripheral axonal pathways and sensilla differentiation. Development 102:869–886
Hartenstein V, Campos-Ortega JA (1984) Early neurogenesis in wildtype Drosophila melanogaster. Rouxs Arch Dev Biol 193:308–325
Hartenstein V, Campos-Ortega JA (1986) The peripheral nervous system of mutants of early neurogenesis in Drosophila melanogaster. Rouxs Arch Dev Biol 195:210–221
Hartenstein V, Jan YN (1992) Studying Drosophila embryogenesis with PlacZ enhancer trap lines. Rouxs Arch Dev Biol 201:194–220
Hartenstein V, Posakony JW (1990) A dual function of the Notch gene in Drosophila sensillum development. Dev Biol 142:13–30
Hartenstein V, Hartenstein A, Rugendorff AE, Green P (1991) The embryonic development of the Drosophila visual system (abstract). 32nd Annual Drosophila Research Conference, Chicago
Hartenstein AY, Rugendorff AE, Tepass U, Hartenstein V (1992) The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development 116:1203–1220
Heitzler P, Simpson P (1991) The choice of cell fate in the epidermis of Drosophila. Cell 64:1083–1092
Hewitt CG (1914) The house-fly (Musca domestica)
Hofbauer A (1979) Die Entwicklung der optischen Ganglien bei Drosophila melanogaster. Inaugural-Dissertation, University of Freiburg i.Br.
Hofbauer A, Campos-Ortega JA (1990) Proliferation pattern and early differentiation of the optic lobes in Drosophila melanogaster. Rouxs Arch Dev Biol 198:264–274
Hoppe PE, Greenspan RJ (1990) The Notch locus of Drosophila is required in epidermal cells for epidermal development. Development 109:875–885
Jürgens G, Lehmann R, Schardin M, Nüsslein-Volhard C (1986) Segmental organisation of the head in the embryo of Drosophila melanogaster. Rouxs Arch Dev Biol 195:359–377
Kidd S, Kelley MR, Young MW (1986) Sequence of the Notch locus of Drosophila melanogaster: relationship of the encoded protein to mammalian clotting and growth factors. Mol Cell Biol 6:3094–3108
Kidd S, Baylies MK, Gasic GP, Young MW (1989) Structure and distribution of the Notch protein in developing Drosophila. Genes Dev 3:1113–1129
Lehmann R, Jimenez F, Dietrich U, Campos-Ortega JA (1983) On the phenotype and development of mutants of early neurogenesis in Drosophila melanogaster. Rouxs Arch Dev Biol 192:62–74
Lindsley DL, Grell EH (1968) Genetic variations of Drosophila melanogaster. (Publication no 627) Carnegie Institution of Washington
Madhavan MM, Schneiderman HA (1977) Histological analysis of the dynamics of growth of imaginal discs and histoblast nests during larval development of Drosophila melanogaster. Rouxs Arch Dev Biol 183:269–305
Panayotou G, End P, Aumailley M, Timpl R, Engel J (1989) Domains of laminin with growth-factor activity. Cell 56:93–101
Paulus HF (1989) Das Homologisieren in der Feinstrukturforschung: das Bolwig Organ der höheren Dipteren und seine Homologisierung mit Stemmata und Ommatidien eines ursprünglichen Facettenauges der Mandibulata. Zool Beitr NF 32:437–478
Pollock JA, Benzer S (1988) Transcript localization of four opsin genes in the three visual organs of Drosophila melanogaster, RH2 is ocellus specific. Nature 333:779–782
Poulson DF (1950) Histogenesis, organogenesis, and differentiation in the embryo of Drosophila melanogaster (Meigen). In: Demerec M (ed) Biology of Drosophila. Wiley, New York, pp 168–274
Ready DF, Hanson TE, Benzer S (1976) Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol 53:217–240
Ruohola H, Bremer KA, Beker D, Swedlow JR, Jan LY, Jan YN (1991) Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell 66:433–439
Shellenbarger DL, Mohler JD (1975) Temperature sensitive mutations of the Notch locus in Drosophila melanogaster. Genetics 81:143–162
Steller H, Fischbach KF, Rubin G (1987) Disconnected: a locus required for neuronal pathway formation. Cell 50:1139–1153
Tepass U, Theres C, Knust E (1990) crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 61:787–799
Tix S, Minden J, Technau GM (1987) Pre-existing neuronal pathways in the developing optic lobes of Drosophila. Development 105:739–746
Turner FR, Mahowald AP (1979) Scanning electron microscopy of Drosophila embryogenesis. III. Formation of the head and caudal segments. Dev Biol 68:96–109
Wharton KA, Johansen KM, Xu T, Artavanis-Tsakonas S (1985) Nucleotide sequence from the neurogenic locus Notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell 43:567–581
White K, Kankel DR (1978) Patterns of cell division and cell movement in the formation of the imaginal nervous system in Drosophila melanogaster. Dev Biol 65:296–321
Zipursky SI, Venkatesh TR, Teplow DB, Benzer S (1984) Neuronal development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell 36:15–26
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Green, P., Hartenstein, A.Y. & Hartenstein, V. The embryonic development of the Drosophila visual system. Cell Tissue Res 273, 583–598 (1993). https://doi.org/10.1007/BF00333712
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00333712