Summary
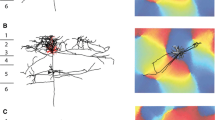
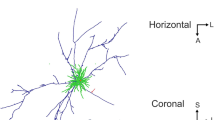
There are numerous hypotheses for the role of the axon collaterals of pyramidal cells. Most hypotheses predict that pyramidal cells activate specific classes of postsynaptic cells. We have studied the postsynaptic targets of two layer III pyramidal cells, that were of special interest because of their clumped axon arborization near, and also 0.4–1.0 mm from the cell body, in register in both layers III and V. 191 terminations from four sites (layers III and V, both in the column of the cell and in distant clumps) were analysed by electron microscopy. Only one bouton contacted a cell body and that was immunoreactive for GABA. The major targets were dendritic spines (84 and 87%), and the remainder were dendritic shafts. Of these 13 were classed as pyramidal-like (P), 8 smooth cell-like (S) and three could not be classified. Four of five S types, but none of the seven P types tested were immunoreactive for GABA, supporting the fine structural classification. The putative inhibitory cells therefore formed not more than 5% of the postsynaptic targets, and their activation could only take place through the convergence of pyramidal cells onto a select population of GABA cells. The results show that the type of pyramidal cells with clumped axons studied here make contacts predominantly with other pyramidal cells. Thus the primary role of both the intra and intercolumnar collateral systems is the activation of other excitatory cells.
Similar content being viewed by others
References
Adams JC (1981) Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem 29: 775
Albus K (1975) A quantitative study of the projection area of the central and the paracentral visual field in area 17 of the cat. I. The precision of the topography. Exp Brain Res 24: 159–179
Baughman RW, Gilbert CD (1981) Aspartate and glutamate as possible neurotransmitters in the visual cortex. J Neurosci 1: 427–439
Bullier J, Kennedy H, Salinger W (1984) Branching and laminar origins of projections between visual cortical areas in the cat. J Comp Neurol 228: 329–341
Creutzfeldt OD, Maekawa K, Hösli L (1969) Forms of spontaneous and evoked postsynaptic potentials of cortical nerve cells. Progr Brain Res 31: 265–273
Creutzfeldt OD, Kuhnt U, Benevento L (1974a) An intracellular analysis of visual cortical neurones to moving stimuli: responses in a co-operative neuronal network. Exp Brain Res 21: 251–274
Creutzfeldt OD, Innocenti GM, Brooks D (1974b) Vertical organization in the visual cortex (area 17). Exp Brain Res 21: 315–336
Creutzfeldt OD, Garey LJ, Kuroda R, Wolff J-R (1977) The distribution of degenerating axons after small lesions in the intact and isolated visual cortex of the cat. Exp Brain Res 27: 419–440
Diamond J, Gray EG, Yasargil GM (1970) The function of the dendritic spine: an hypothesis. In: Anderson P, Jansen JKS (eds) Excitatory synaptic mechanisms. Universitets Forlaget, Oslo, pp 213–222
Emson PC, Lindvall O (1979) Distribution of putative neurotransmitters in the neocortex. Neuroscience 4: 1–30
Feldman ML (1984) Morphology of the neocortical pyramidal neuron. In: Peters A, Jones EG (eds) Cerebral cortex: cellular components of the cerebral cortex, Vol. I. Plenum Press New York London, pp 123–200
Ferster D, Lindström S (1983) An intracellular analysis of geniculo-cortical connectivity in area 17 of the cat. J Physiol 342: 181–215
Ferster D, Lindström S (1985) Synaptic excitation of neurones in area 17 of the cat by intracortical axon collaterals of corticogeniculate cells. J Physiol 367: 233–252
Fisken RA, Garey LJ, Powell TPS (1975) The intrinsic association and comissural connections of area 17 of the visual cortex. Philos Trans R Soc Lond B 272: 487–536
Freund TF, Martin KAC, Smith AD, Somogyi P (1983) Glutamate decarboxylase-immunoreactive terminals of Golgi-impregnated axo-axonic cells and of presumed basket cells in synaptic contact with pyramidal cells of the cat's visual cortex. J Comp Neurol 221: 263–278
Freund TF, Martin KAC, Somogyi P, Whitteridge D (1985) Innervation of cat visual areas 17 and 18 by physiologically identified X- and Y-type thalamic afferents. IL Identification of postsynaptic targets by GABA immunocytochemistry and Golgi impregnation. J. Comp Neurol 242: 275–291
Friedlander MJ, Lin C-S, Sherman SM (1979) Structure of physiologically identified X and Y cells in the cat's lateral geniculate nucleus. Science 204: 1114–1117
Gilbert CD (1985) Horizontal integration in the neocortex. Trends Neurosci 8: 160–165
Gilbert CD, Kelly JP (1975) The projections of cells in different layers of the cat's visual cortex. J Comp Neurol 163: 81–106
Gilbert CD, Wiesel TN (1979) Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature 280: 120–125
Gilbert CD, Wiesel TN (1983) Clustered intrinsic connections in cat visual cortex. J Neurosci 3: 1116–1133
Hanker JS, Yates PE, Metz CB, Rustioni A (1977) A new specific, sensitive and non-carcinogenic agent for the demonstration of horseradish peroxidase. Histochem J 9: 789–792
Hodgson AJ, Penke B, Erdei A, Chubb IW, Somogyi P (1985) Antiserum to γ-amino-butyric acid. I. Production and characterisation using a new model system. J Histochem Cytochem 33: 229–239
Hubel DH, Wiesel TN (1962) Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol 160: 106–154
Hubel DH, Wiesel TN (1963) Shape and arrangement of columns in cat's striate cortex. J Physiol 165: 559–568
Hubel DH, Wiesel TN (1968) Receptive fields and functional architecture of monkey striate cortex. J Physiol 195: 215–243
Hubel DH, Wiesel TN (1977) Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B 198: 1–59
Jack JJB, Noble D, Tsien RW (1975) Electric current flow in excitable cells. Oxford University Press, Oxford, pp 197–222
Landry P, Labelle A, Deschenes M (1980) Intracortical distribution of axonal collaterals of pyramidal tract cells in the cat motor cortex. Brain Res 191: 327–336
Lorente de Nó R (1922) La corteza cerebral del raton. I. La corteza acustica. Trab Lab Invest Biol Univ Madrid. 20: 41–78
Lund JS (1973) Organisation of neurons in the visual cortex, area 17, of the monkey (Macaca mulatta). J Comp Neurol 147: 455–496
Lund JS (1984) Spiny stellate neurons. In: Peters A, Jones EG (eds) Cerebral cortex, cellular components of the cerebral cortex, Vol. I. Plenum Press, New York London, pp 255–308
Lund JS, Boothe R (1975) Interlaminar connections and pyramidal neuron organization in the visual cortex, area 17, of the macaque monkey. J Comp Neurol 159: 305–334
Lund JS, Henry GH, Mac Queen CL, Harvey AR (1979) Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. J Comp Neurol 184: 599–618
Martin KAC (1984) Neuronal circuits in cat striate cortex. In: Jones EG, Peters A (eds) Cerebral cortex, functional properties of cortical cells, Vol II. Plenum Press, New York London, pp 241–284
Martin KAC, Somogyi P (1985) Local excitatory circuits in area 17 of the cat. In: Rose D, Dobson VG (eds) Models of the visual cortex. John Wiley & Sons New York, pp 504–513
Martin KAC, Somogyi P, Whitteridge D (1983) Physiological and morphological properties of identified basket cells in the cat's visual cortex. Exp Brain Res 50: 193–200
Martin KAC, Whitteridge D (1984) Form, function, and intracortical projections of spiny neurones in the striate visual cortex of the cat. J Physiol (Lond) 353: 463–504
Matsubara J, Cynader M, Swindale NV, Stryker MP (1985) Intrinsic projections within visual cortex: evidence for orientation-specific local connections. Proc Natl Acad Sci USA 82: 935–939
McGuire BA, Gilbert CD, Wiesel TN (1985) Ultrastructural characterization of long-range clustered horizontal connections in monkey striate cortex. Soc Neurosci Abstr 11: 17
McGuire BA, Hornung J-P, Gilbert CD, Wiesel TN (1984) Patterns of synaptic input to layer 4 of cat striate cortex. J Neurosci 4: 3021–3033
Mitchinson G, Crick F (1982) Long axons within the striate cortex — their distribution, orientation, and patterns of connection. Proc Natl Acad Sci USA 79: 3661–3665
Mitzdorf U, Singer W (1978) Prominent excitatory pathways in the cat visual cortex (A17 and A18): a current source density analysis of electrically evoked potentials. Exp Brain Res 33: 371–394
Mitzdorf J, Singer W (1979) Excitatory synaptic ensemble properties in the visual cortex of the macaque monkey: a current source density analysis of electrically evoked potentials. J Comp Neurol 187: 71–84
Nelson JI, Frost BJ (1985) Intracortical facilitation among co-orientated co-axially aligned simple cells in cat striate cortex. Exp Brain Res 61: 54–61
Noda T, Yamamoto T (1984) Response properties and morphological identification of neurons in the cat motor cortex. Brain Res 306: 197–206
Peters A, Saint-Marie RL (1984) Smooth and sparsely spinous nonpyramidal cells forming local axonal plexuses. In: Peters A, Jones EG (eds) Cerebral cortex: cellular components of the cerebral cortex, Vol I. Plenum Press New York London, pp 419–445
Phillips CG (1959) Actions of antidromic pyramidal volleys on single Betz cells in the cat. QJ Exp Physiol 44: 1–25
Pongracz F (1985) The function of dendritic spines: a theoretical study. Neuroscience 15: 933–946
Ramón y Cajal S (1899) Estudios sobre la corteza cerebral humana. Corteza visual. Rev Trim Microgr 4: 1–63
Ribak CE (1978) Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase J Neurocytol 7: 461–478
Rockland KS, Lund JS (1982) Widespread periodic intrinsic connections in the tree shrew visual cortex. Science 215: 1532–1534
Rockland KS, Lund JS, Humphrey AL (1982) Anatomical banding of intrinsic connections in striate cortex of tree shrews (Tupaia glis). J Comp Neurol 209: 41–58
Scheibel ME, Scheibel AB (1970) Elementary processes in selected thalamic and cortical subsystems — the structural substrates. In: Schmitt FO (eds) The neurosciences second study program. The Rockefeller University Press, New York, pp 443–457
Segraves MA, Innocenti GM (1985) Comparison of the distributions of ipsilaterally and contralaterally projecting corticocortical neurons in cat visual cortex using two fluorescent tracers. J Neurosci 5: 2107–2118
Sholl DA (1955) The organization of the visual cortex in the cat. J Anat 89: 33–46
Sillito AM (1979) Inhibitory mechanisms influencing complex cell orientation selectivity and their modification at high resting discharge levels. J Physiol 289: 33–53
Sillito AM (1984) Functional considerations of the operation of GABAergic inhibitory processes in the visual cortex. In: Jones EG, Peters A (eds) Cerebral cortex, functional properties of cortical cells, Vol II. Plenum Press, New York London, pp 91–117
Somogyi P (1978) The study of Golgi stained cells and of experimental degeneration under the electron microscope: a direct method for the identification in the visual cortex of three successive links in a neuron chain. Neuroscience 3: 167–180
Somogyi P, Kisvárday ZF, Martin KAC, Whitteridge D (1983) Synaptic connections of morphologically identified and physiologically characterized large basket cells in the striate cortex of cat. Neuroscience 10: 261–294
Somogyi P, Freund TF, Hodgson AJ, Somogyi J, Beroukas D, Chubb IW (1985a) Identified axo-axonic cells are immunoreactive for GABA in the hippocampus and visual cortex of the cat. Brain Res 332: 143–149
Somogyi P, Hodgson AJ, Chubb IW, Penke B, Erdei A (1985b) Antiserum to γ-amino-butyric acid. II. Immunocytochemical application to the central nervous system. J Histochem Cytochem 33: 240–248
Somogyi P, Hodgson AJ (1985) Antiserum to γ-amino-butyric acid. III. Demonstration of GABA in Golgi-impregnated neurons and in conventional electron microscopic sections of cat striate cortex. J Histochem Cytochem 33: 249–257
Stefanis C, Jasper H (1964) Recurrent collateral inhibition in pyramidal tract neurones. J Neurophysiol 27: 855–877
Szentágothai J (1965) The use of degeneration methods in the investigation of short neuronal connexions. In: Singer M, Schade JP (eds) Degeneration patterns in the nervous system. Progress in Brain Research, Vol 14, pp 1–32
Szentágothai J (1973) Synaptology of the visual cortex. In: Jung R (ed) Handbook of sensory physiology, Vol VII. Springer, Berlin Heidelberg New York, pp 270–321
Szentágothai J (1975) The ‘module-concept’ in cerebral cortex architecture. Brain Res 95: 475–496
Szentágothai J (1978) The neuron network of the cerebral cortex: a functional interpretation. The Ferrier Lecture, 1977. Proc R Soc Lond B 201: 219–248
Szentágothai J (1979) Local neuron circuits of the neocortex. In: Schmitt FO, Worden FG (eds) The Neurosciences Fourth Study Program, pp 399–415
Ts'o DY, Gilbert CD, Wiesel TN (1986) Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J Neurosci 6: 1160–1170
Winfield DA, Brooke RNL, Sloper JJ, Powell TPS (1981) A combined Golgi-electron microscopic study of the synapses made by the proximal axon and recurrent collaterals of a pyramidal cell in the somatic sensory cortex of the monkey. Neuroscience 6: 1217–1230
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kisvárday, Z.F., Martin, K.A.C., Freund, T.F. et al. Synaptic targets of HRP-filled layer III pyramidal cells in the cat striate cortex. Exp Brain Res 64, 541–552 (1986). https://doi.org/10.1007/BF00340492
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00340492