Abstract
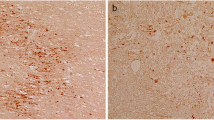
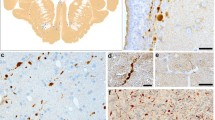
β-Amyloid precursor protein (βAPP) can be detected immunocytochemically at sites of axonal injury in the brain, and has recently been found to be a useful marker for injured axons in patients who survived for only 3 h after head trauma. It is transported by last axonal transport and is thought to accumulate in detectable levels where the cytoskeleton breaks down. If this theory is correct, other substances should accumulate here in the same way, so we have used antibodies to other neuronal proteins to compare their efficacy as markers of axonal injury. SNAP-25, chromogranin A and cathepsin D also marked injured axons at all survival times studied (2.5h–2 weeks), although they were not as sensitive or specific as βAPP. Immunolabelling for the 68-kDa neurofilament subunit (NF68) was present in most uninjured axons, and allowed axonal swellings to be seen in some cases. Synaptophysin, GAP-43, ubiquitin or tau did not label any normal or injured axons in this study. We, therefore, suggest that βAPP should be the immunocytochemical marker of choice for the detection of injured axons. This study also showed that microwave antigen retrieval significantly enhances the immunoreactivity of SNAP-25, chromogranin A, synaptophysin, GAP-43, ubiquitin and tau, in addition to that of βAPP, in formalin-fixed, paraffin-embedded tissue, and reveals NF68 antigenicity where it was not previously detectable.
Similar content being viewed by others
References
Adams JH, Graham DI, Murray LS, Scott G (1982) Diffuse axonal injury due to non-missile head injury in humans. Ann Neurol 12: 557–563
Adams JH, Graham DI, Gennarelli TA, Maxwell WI (1991) Diffuse axonal injury in non-missile head injury. J Neurol Neurosurg Psychiatry 54: 481–483
Adams LA, Ang L-C, Munoz DG (1993) Chromogranin A, a soluble synaptic vesicle protein, is found in cortical neurons other than previously defined peptidergic neurons in the human neocortex. Brain Res 602: 336–341
Barrett AJ (1980) Cathepsin D: the lysosomal aspartic proteinase. In: Barrett AJ (ed) Protein degradation in health and disease, Ciba Foundation symposium, 1979. Excerpta Medica, Amsterdam, pp 37–50
Benowitz LI, Routtenberg A (1987) A membrane phosphoprotein associated with neural development, axonal regeneration, phospholipid metabolism, and synaptic plasticity. Trends Neurosci 10: 527–532
Benowitz LI, Rodriguez W, Pakevich P, Mufson EJ, Schenk D, Neve RL (1989) The amyloid precursor protein is concentrated in neuronal lysosomes in normal and Alzheimer's subjects. Exp Neurol 106: 237–250
Benowitz LI, Perrone-Bizzozero NI, Finkelstein SP, Bird ED (1989) Localisation of the growth-associated phosphoprotein GAP-43 (B-50, F1) in the human cerebral cortex. J Neurosci 9: 990–995
Catteruccia N, Willingdale-Theune J, Bunke D, Prior R, Masters CL, Crisanti A, Beyreuther K (1990) Ultrastructural localisation of the putative precursors of the A4 amyloid protein associated with Alzheimer's disease. Am J Pathol 137: 19–26
Cattoretti G, Pileri S, Parravicini C, Becker MHG, Poggi S, Bifulco C, Key G, D'Amato L, Sabattini E, Feudale E, Reynold F, Gerdes J, Rilke F (1993) Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol 171: 83–98
Cochran E, Bacci B, Chen Y, Patton A, Gambetti P, Autilio-Gambetti L (1991) Amyloid precursor protein and ubiquitin immunoreactivity in dystrophic axons is not unique to Alzheimer's Disease. Am J Pathol 139: 485–489
Crooks DA, Scoltz CL, Vowles G, Greenwald S (1992) Axonal injury in closed head injury by assault: a quantitative study. Med Sci Law 32: 109–117
De Camilli P, Jahn R (1990) Pathways to regulated exocytosis in humans. Annu Rev Physiol 52: 625–645
Ferreira A, Caceras A, Kosik KS (1993) Intraneuronal compartments of the amyloid precursor protein. J Neurosci 13: 3112–3123
Fischer-Colbrie R, Hagn C, Schober M (1987) Chromogranins A, B and C: widespread constituents of secretory vesicles. Ann NY Acad Sci 493: 120–134
Geinisman Y, Bondareff W, Telser A (1977) Transport of [3H]fucose-labeled glycoproteins in the septo-hippocampal pathway of young adult and senescent rats. Brain Res 125: 182–186
Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW (1993) β-amyloid precursor protein (βAPP) as a marker for axonal injury after head injury. Neurosci Lett 160: 139–144
Golde TE, Estus S, Younkin LH, Selkoe DJ, Younkin SG (1992) Processing of the amyloid precursor to potentially amyloidogenic derivatives. Science 255: 728–730
Grady MS, McLaughlin MR, Christman CW, Valadka AB, Fligner CL, Povlishock JT (1993) The use of antibodies targeted against the neurofilament subunits for the detection of diffuse axonal injury in humans. J Neuropathol Exp Neurol 52: 143–152
Gultekin SH, Smith TW (1994) Diffuse axonal injury in craniocerebral trauma: a comparative histologic and immunocytochemical study. Arch Pathol Lab Med 118: 168–171
Haass N, Koo EH, Mellon A, Hung AY, Selkoe DJ (1992) Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature 357: 500–503
Hershko A (1991) The ubiquitin pathway for protein degradation. Trends Biochem Sci 16: 265–268
Joachim C, Games D, Morris J, Ward P, Frenkel D, Selkoe D (1991) Antibodies to non-beta regions of the beta amyloid precursor protein detect a subset of senile plaques. Am J Pathol 138: 373–384
Jortner BS, Scarratt WK, Modransky PD, Perkins SK (1993) Ubiquitin expression in degenerating axons of equine cervical stenotic myelopathy. Soc Neurosci Abstr 765.2
Kawarabayashi T, Shoji M, Yamaguchi H, Tanaka M, Harigaya Y, Ishiguro K, Hirai S (1993) Amyloid β-protein precursor accumulates in swollen neurites throughout rat brain with aging. Neurosci Lett 153: 73–76
Kidd M (1963) Paired helical filaments in electron microscopy of Alzheimer's disease. Nature 197: 192–193
Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyruther K, Fischer P, Masters CL, Price DL (1990) Precursor of amyloid protein in Alzheimer's disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci USA 87: 1561–1565
Lee VM-Y, Balin BJ, Otvos L Jr, Trojanowski JQ (1991) A major subunit of paired helical filaments and derivatised forms of normal tau. Science 251: 675–678
Li J-Y, Kling-Petersen A, Dahlstrom A (1993) GAP 43-like immunoreactivity in normal adult rat sciatic nerve, spinal cord, and motoneurons: axonal transport and effect of spinal cord transection. Neuroscience 57: 759–776
Martin JE, Mather KS, Swash M, Garofalo O, Dale GE, Leigh PN, Anderton BBH (1990) Spinal cord trauma in man: expression. Brain 113: 1553–1562
Munoz D (1991) Chromogranin A-like immunoreactive neurites are major constituents of senile plaques. Lab Invest 64: 826–832
Ohgami T, Kitamoto T, Tateishi J (1992) Alzheimer's amyloid precursor protein accumulates within axonal swellings in human brain lesions. Neurosci Lett 136: 75–78
Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsby M, Bloom FE, Wilson MC (1989) The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol 109: 3039–3052
Pappola MA, Omar R, Saran B (1989) The ‘normal’ brain. ‘Abnormal’ ubiquitiated deposits highlight an age-related protein change. Am J Pathol 135: 585–591
Povlishock JT (1992) Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol 2: 1–12
Reid WA, Valler MJ, Kay J (1986) Immunolocalisation of cathepsin D in normal and neoplastic human tissues. J Clin Pathol 39: 1323–1330
Roberts GW, Gentleman SM, Lynch A, Murray L, Landon M, Graham DI (1994) β-Amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer's disease. J Neurol Neurosurg Psychiatry 57: 419–425
Schubert W, Prior R, Weidemann A, Dircksen H, Multhaup G, Masters C, Beyreuther K (1991) Localisation of Alzheimer βA4 amyloid precursor protein at central and peripheral synaptic sites. Brain Res 563: 184–194
Schweitzer JB, Park MR, Einhaus SL, Robertson JT (1993) Ubiquitin marks the reactive swellings of diffuse axonal injury. Acta Neuropathol 85: 503–507
Selkoe DJ (1993) Physiological production of the b-amyloid protein and the mechanism of Alzheimer's disease. Trends Neurosci 16: 403–409
Sherriff FE, Bridges LR, Sivaloganathan S (1994) Early detection of axonal injury after human head trauma using immunocytochemistry for β-amyloid precursor protein. Acta Neuropathol 87: 55–62
Sherriff FE, Bridges LR, Jackson P (1994) Microwave antigen retrieval enhances β-amyloid precursor protein immunoreactivity in formalin-fixed brain in Alzheimer's disease and after head injury. Neuroreport 5: 1085–1088
Skene JHP, Jacobson RD, Snipes GJ, McGuire CB, Norden JJ, Freeman JA (1987) A protein induced during nerve growth (GAP-43) is a major component of growth cone membranes. Science 223: 783–786
Snipes GJ, Chan SY, McGuire CB, Costello BR, Norden JJ, Freeman JA, Routtenberg A (1987) Evidence for the coidentification of GAP-43, a growth-associated protein, and, F1, a plasticity-associated protein. J Neurosci 7: 4066–4075
Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Germanos S, Tempst P, Rothman JE (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature 362: 318–324
Somogyi P, Hodgson AJ, DePotter RW, Fischer-Colbrie R, Schober M, Winkler H, Chubb IW (1984) Chromogranin immunoreactivity in the central nervous system, immunocytochemical characterisation and relationship to catecholamine and enkephalin pathways. Brain Res Rev 8: 193–230
Tomlinson DR (1975) Two populations of granular vesicles in constricted post-gangloinic sympathetic nerves. J Physiol (Lond) 245: 727–735
Tsukita S, Ishikawa H (1980) The movement of membraneous organelles in axons, electron microscopic identification of anterogradely and retrogradely transported organelles. J Cell Biol 84: 513–530
Viancour TA, Kreiter NA (1993) Vesicular fast axonal transport rates in young and old rat axons. Brain Res 628: 209–217
Weidenmann B, Franke WW (1985) Identification and localisation of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell 41: 1017–1028
Winkler H, Fischer-Colbrie R (1990) Common membrane proteins of chromaffin granules, endocrine and synaptic vesicles: properties, tissue distribution, membrane topography and regulation of synthesis. Neurochem Int 17: 245–262
Wisniewski HM, Narang HK, Corsellis JAN, Terry RD (1976) Ultrastructural studies of the neuropil and neurofibrillary tangles in Alzheimer's disease and post-traumatic dementia. J Neuropathol Exp Neurol 35: 367
Yaghmai A, Povlishock JT (1992) Traumatically induced reactive change as visualised through the use of monoclonal antibodies targeted to neurofilament subunits. J Neuropathol Exp Neurobiol 51: 158–176
Author information
Authors and Affiliations
Additional information
Supported by the Home Office Policy Advisory Board for Forensic Pathology (UK)
Rights and permissions
About this article
Cite this article
Sherriff, F.E., Bridges, L.R., Gentleman, S.M. et al. Markers of axonal injury in post mortem human brain. Acta Neuropathol 88, 433–439 (1994). https://doi.org/10.1007/BF00389495
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00389495