Abstract
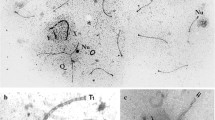
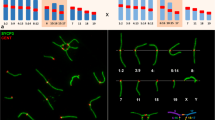
Complex Robertsonian translocation heterozygosities in the mouse have been used to test different hypotheses regarding the correlation between male hybrid sterility and chromosomal abnormality. Synaptonemal complexes of meiotic super-chains and super-rings involving 15 to 18 metacentric chromosomes were studied in relation to spermatogenic histology. Both types of multivalents showed a characteristic pachytene pattern of alternating paired and non-paired segments. The amount of unpaired segments in rings was about 18% and in chains about 23% of the total length of multivalent chromosomes. The meiotic chains were associated with the proximal part of the X chromosomes in more than 60% of pachytene cells; a similar tight proximity of rings with X or Y chromosomes was never found. Complete arrest of germ cell maturation correlated with super-chains and inconspicuous testicular histology with super-rings. This demonstrates that an excessive amount of unpaired chromosomal axes does not leadper se to male infertility through gametogenic breakdown. On the contrary, the results clearly indicate spermatogenic impairment in this system of multimetacentric heterozygosity as a reflection of X chromosome super-chain interference.
Similar content being viewed by others
References
de Boer P, Searle AG, van der Hoeven FA, de Rooij DG, Beechey CV (1986) Male pachytene pairing in single and double translocation heterozygotes and spermatogenic impairment in the mouse.Chromosoma 93: 326–336.
de Boer P, de Jong JH (1989) Chromosome pairing and fertility in mice. In: Gillies CB, ed.Fertility and Chromosome Pairing: Recent Studies in Plants and Animals. Boca Raton: CRC Press, pp 37–76.
Burgoyne PS, Baker TG (1984) Meiotic pairing and gametogenic failure. In: Evans CW, Dickinson HG, eds.Controlling Events in Meiosis. Proc 38th SEB Symp, Reading, England. Cambridge: Company of Biologists, pp 349–362.
Burgoyne PS, Mahadevaiah S, Mittwoch U (1985) A reciprocal autosomal translocation which causes male sterility in the mouse also impairs oogenesis.J Reprod Fert 75: 647–652.
Burgoyne PS, Mahadevaiah SK (1993) Unpaired sex chromosomes and gametogenic failure. In: Sumner AT, Chandley AC, eds.Chromosomes Today, Vol. 11. London: Chapman and Hall, pp 243–263.
Chandley AC, Speed RM, McBeath S, Hargreave TB (1986) A human 9:20 reciprocal translocation associated with male infertility analyzed at prophase and metaphase I of meiosis.Cytogenet Cell Genet 41: 145–153.
Dresser ME, Moses MJ (1980) Synaptonemal complex karyotyping in spermatocytes of the Chinese hamster (Cricetulus griseus). IV. Light and electron microscopy of synapsis and nucleolar development by silver staining.Chromosoma 76: 1–22.
Forejt J (1979) Meiotic studies of translocations causing male sterility in the mouse. II. Double heterozygotes for Robertsonian translocations.Cytogenet Cell Genet 23: 163–170.
Forejt J (1985) Chromosomal and genic sterility of hybrid type in mice and men.Exp Clin Immunogenet 2: 106–119.
Forejt J, Gregorova S (1977) Meiotic studies of translocations causing male sterility in the mouse. I. Autosomal reciprocal translocations.Cytogenet Cell Genet 19: 159–179.
Gabriel-Robez O, Ratomponirina C, Dutrillaux B, Carre-Pigeon F, Rumpler Y (1986) Meiotic association between the XY chromosomes and the autosomal quadrivalent of a reciprocal translocation in two infertile men, 46,XY,t(19;22) and 46,XY,t(17;21).Cytogenet Cell Genet 43: 154–160.
Garagna S, Redi CA, Zucotti M, Britton-Davidian J, Winking H (1990) Kinetics of oogenesis in mice heterozygous for Robertsonian translocation.Differentiation 42: 167–171.
Gropp A, Winking H (1981) Robertsonian translocations: cytology, meiosis, segregation patterns and biological consequences of heterozygosity. In: Berry RJ, ed.Biology of the House Mouse. London: Academic Press, pp 141–181.
Gropp A, Winking H, Redi C (1982) Consequences of Robertsonian heterozygosity: segregational impairment of fertility versus male-limited sterility. In: Crosignani PG, Rubin BL, Fraccaro M, eds.Genetic Control of Gamete Production and Function. London; Academic Press: New York; Grune and Stratton, pp 115–134.
Guichaoua MR, Speed RM, Luciani JM, Delafontaine D, Chandley AC (1992) Infertility in human males with autosomal translocations. II. Meiotic studies in three reciprocal rearrangements, one showing tertiary monosomy in a 45-chromosome individual and his father.Cytogenet Cell Genet 60: 96–101.
Hotta Y, Chandley AC (1982) Activities of X-linked enzymes in spermatocytes of mice rendered sterile by chromosomal alterations.Gamete Res 6: 65–72.
Johannisson R, Gropp A, Winking H, Coerdt W, Rehder H, Schwinger E (1983) Down's syndrome in the male. Reproductive pathology and meiotic studies.Hum Genet 63: 132–138.
Johannisson R, Löhrs U, Wolff HH, Schwinger E (1987) Two different XY-quadrivalent associations and impairment of fertility in men.Cytogenet Cell Genet 45: 222–230.
Johannisson R, Löhrs U, Passarge E (1988) Pachytene analysis in males heterozygous for a familial translocation (9;12;13) (q22;q22;q32) ascertained through a child with partial trisomy 9.Cytogenet Cell Genet 47: 160–166.
Johannisson R, Schwinger E, Wolff HH, vom Ende V, Löhrs U (1993) The effect of 13:14 Robertsonian translocations on germ-cell differentiation in infertile males.Cytogenet Cell Genet 63: 151–155.
Kovacs A, Villagomez DAF, Gustavsson I, Lindblad K, Foote RH, Howard TH (1992) Synaptonemal complex analysis of a three-breakpoint translocation in a subfertile bull.Cytogenet Cell Genet 61: 195–201.
Lifschytz E, Lindsley DL (1972) The role of X-chromosome inactivation during spermatogenesis.Proc Natl Acad Sci USA,69: 182–186.
McCarrey JR, Dilworth DD (1992) Expression ofXist in mouse germ cells correlates with X-chromosome inactivation.Nature Genet 2: 200–203.
McKee BD, Handel MA (1993) Sex chromosomes, recombination, and chromatin conformation.Chromosoma 102: 71–80.
Miklos GLG (1974) Sex-chromosome pairing and male fertility.Cytogenet Cell Genet 13: 558–577.
Mittwoch U, Mahadevaiah SK, Setterfield LA (1990) Pachytene pairing and oocyte numbers in mice with two single Robertsonian translocations and the male-sterile compound with monobrachial homology.Cytogenet Cell Genet 53: 144–147.
Mittwoch U, Mahadevaiah SK (1992) Unpaired chromosomes at meiosis: causes or effect of gametogenic insufficiency?Cytogenet Cell Genet 59: 274–279.
Moses MJ (1980) New cytogenetic studies on mammalian meiosis. In: Serio M, Martini L, eds.Animal Models in Human Reproduction. New York: Raven Press, pp 169–190.
Richler C, Soreq H, Wahrman J (1992) X inactivation in mammalian testis is correlated with inactive X-specific transcription.Nature Genet 2: 192–195.
Rosenmann A, Wahrman J, Richler C, Voss R, Persitz A, Goldman B (1985) Meiotic association between the XY chromosomes and unpaired autosomal elements as a cause of human male sterility.Cytogenet Cell Genet 39: 19–29.
Saadallah N, Hulten M (1985) A complex three breakpoint translocation involving chromosomes 2, 4, and 9 identified by meiotic investigations of a human male ascertained for subfertility.Hum Genet 71: 312–320.
Salido EC, Yen PH, Mohandas TK, Shapiro LJ (1992) Expression of the X-inactivation-associated geneXIST during spermatogenesis.Nature Genet 2: 196–199.
Schmid M, Johannisson R, Haaf T, Neitzel H (1987) The chromosomes ofMicromys minutus (Rodentia, Murinae). II. Pairing pattern of X and Y chromosomes in meiotic prophase.Cytogenet Cell Genet 45: 121–131.
Setterfield LA, Mahadevaiah S, Mittwoch U (1988) Chromosome pairing and germ cell loss in male and female mice carrying a reciprocal translocation.J Reprod Fert 82: 369–379.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Johannisson, R., Winking, H. Synaptonemal complexes of chains and rings in mice heterozygous for multiple Robertsonian translocations. Chromosome Res 2, 137–145 (1994). https://doi.org/10.1007/BF01553492
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01553492