Summary
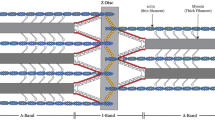
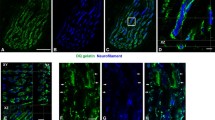
The Z-disc of striated muscle is degraded by the Ca2+ -activated proteinase, calpain, during autolysis of muscle fibres. The effect of calpain on proteins in preparations of Z-discs isolated fromLethocerus flight muscle has been studied. Calpain releasesα-actinin from the Z-disc and digests two hydrophobic proteins associated with the Z-disc, zeelin 1 (35 kD) and zeelin 2 (23 kD). The Ca2+ sensitivity of zeelin digestion is shifted to lower Ca2+ concentrations (within the physiological range) in the presence of the phospholipids phosphatidyl inositol or phosphatidyl choline and diacylglycerol. The release ofα-actinin is not affected by phospholipid. Preparations of isolated Z-discs have five times as much associated phospholipid (w/w) as myofibrils and the composition of the lipid differs from that of myofibrils. In muscle fibres the action of calpain on zeelins may be controlled by the composition of phospholipid in the fibres as well as by Ca2+.
Similar content being viewed by others
References
Azana, J.-L., Raymond, J., Robin, J.-M., Cottin, P. &Ducastaing, A. (1979) Purification and some physico-chemical and enzymatic properties of a calcium ionactivated neutral proteinase from rabbit skeletal muscle.Biochem. J. 183, 339–47.
Bangham, A. D. &Horne, R. W. (1964) Negative staining of phospholipids and their structural modifications by surface-active agents as observed in the electron microscope.J. molec. Biol. 8, 660–8.
Baron, M. D., Davison, M. D., Jones, P. &Critchley, D. R. (1987) The sequence of chick α-actinin reveals homologies to spectrin and calmodulin.J. Biol. Chem. 262, 17623–9.
Berridge, M. J. (1988) Inositol lipids and calcium signalling.Proc. Roy. Soc. Ser. B 234, 359–78.
Bullard, B., Bell, J., Craig, R. &Leonard, K. (1985) Arthrin: a new actin-like protein in insect flight muscle.J. molec. Biol. 182, 443–54.
Bullard, B., Dabrowska, R. &Winkelman, L. (1973) The contractile and regulatory proteins of insect flight muscle.Biochem. J. 135, 277–86.
Bullard, B. &Reedy, M. K. (1973) How many myosins per crossbridge? II Flight muscle myosin from the blowflySarcophaga bullata.Cold Spring Harb. Symp. quant. Biol. 37, 423–8.
Busch, W. A., Stromer, M. H., Goll, D. E. &Suzuki, A. (1972) Ca2+ -specific removal of Z-lines from rabbit skeletal muscle.J. Cell Biol. 52, 367–81.
Cong, J., Goll, D. E., Peterson, A. M. &Kapprell, H. P. (1989) The role of autolysis in activity of the Ca2+ -dependent proteinases (μ-calpain and m-calpain).J. Biol. Chem. 264, 10096–103.
Coolican, S. A. &Hathaway, D. R. (1984) Effect of L-a-phosphatidylinositol on a vascular smooth muscle Ca2+ -dependent protease. Reduction of the Ca2+ requirement of autolysis.J. Biol. Chem. 259, 11627–30.
Dayton, W. R., Reville, W. J., Goll, D. E. &Stromer, M. H. (1976) A Ca2+-activated protease possibly involved in myofibrillar protein turnover.Biochemistry 15, 2159–67.
Dayton, W. R. &Schollmeyer, J. V. (1980) Isolation from porcine cardiac muscle of a Ca2+ -activated protease that partially degrades myofibrils.J. molec. cell. Cardiol. 12, 533–51.
Dayton, W. R., Schollmeyer, J. V., Lepley, R. A. &Cortes, L. R. (1981) A calcium-activated protease possibly involved in myofibrillar protein turnover. Isolation of a low-calcium-requiring form of the protease.Biochim. Biophys. Acta 659, 48–61.
Goll, D. E., Edmunds, T., Kleese, W. C., Sathe, S. K. &Shannon, J. D. (1985) Some properties of the Ca2+ -dependent proteinase.Prog. Clin. Biol. Res. 180, 151–64.
Goll, D. E., Shannon, J. D., Edmunds, T., Sathe, S. K., Kleese, W. C. &Nagainis, P.A. (1983) Properties and regulation of the Ca2+-dependent proteinase. InCalcium-Binding Proteins (edited byDe Bernard, B., Sottocasa, G. L., Sandri, G., Carafoli, E., Taylor, A. N., Vanaman, T. C. &Williams, R. J. P.), pp. 19–35. Amsterdam: Elsevier Science Publishers.
Gopalakrishna, R. &Barsky, S. H. (1986) Hydrophobic association of calpains with subcellular organelles. Compartimentalization of calpains and the endogenous inhibitor calpastatin in tissues.J. Biol. Chem. 261, 13936–42.
Haeberle, J. R., Coolican, S. A., Evan, A. &Hathaway, D. R. (1985) The effects of a calcium-dependent protease on the ultrastructure and contractile mechanics of skinned uterine smooth muscle.J. Musc. Res. Cell Motil. 6, 347–63.
Hathaway, D. R., Werth, D. K. &Haeberle, J. R. (1982) Limited autolysis reduces the Ca2+-requirement of a smooth muscle Ca2+-activated protease.J. Biol. Chem. 257, 9072–7.
Imajoh, S., Kawasaki, H. &Suzuki, K. (1986) The amino-terminal hydrophobic region of the small subunit of calcium-activated neutral protease (CANP) is essential for its activation by phosphatidylinositol.J. Biochem. 99, 1281–4.
Kishimoto, A., Kajikawa, N., Shiota, M. &Nishizuka, Y. (1983) Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease.J. Biol. Chem. 258, 1156–64.
Kishimoto, A., Takai, Y., Mori, T., Kikkawa, U. &Nishizuka, Y. (1980) Activation of calcium and phopholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover.J. Biol. Chem. 255, 2273–76.
Laemmli, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature 227, 680–5.
Martonosi, A. N. &Beeler, T. J. (1983) Mechanism of Ca2+ transport by sarcoplasmic reticulum. InHandbook of Physiology, Skeletal Muscle (edited byPeachey, L. D., Adrian, R. H. &Geiger, S. R.), Vol. 10, pp. 417–85. Bethesda: American Physiol. Soc.
McClare, C. W. F. (1971) An accurate and convenient organic phosphorus assay.Anal. Biochem. 39, 527–30.
Mellgren, R. L. (1980) Canine cardiac calcium-dependent proteases: Resolution of two forms with different requirements for calcium.FEBS Lett. 109, 129–33.
Mellgren, R. L. (1987) Calcium-dependent proteases: an enzyme system active at cellular membranes?FASEB J. 1, 110–5.
Miller, N. G. A., Hill, M. W. &Smith, M. W. (1976) Positional and species analysis of membrane phospholipids extracted from goldfish adapted to different environmental temperatures.Biochim. Biophys. Acta 455, 644–54.
Muguruma, M., Muguruma, Y. &Fukazawa, T. (1980) Contribution of Z-line constituents to the formation of the contraction bands of chicken myofibrils on addition of Mg2+-ATP.J. Biochem. 88, 145–9.
Murachi, T., Tanaka, K., Hatanaka, M. &Murakami, T. (1981) Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin).Adv. Enzyme Regul. 19, 407–24.
Nishizuka, Y. (1984) The role of protein kinase C in cell surface signal transduction and tumour promotion.Nature 308, 693–8.
Papahadjopoulos, D. &Miller, N. (1967) Phospholipid model membranes I. Structural characteristics of hydrated liquid crystals.Biochim. Biophys. Acta 135, 624–38.
Pérrin, D. D. &Sayce, I. G. (1967) Computer calculation of the equilibrium concentrations of metal ions and complexing species.Talanta 14, 833–42.
Pontremoli, S., Melloni, E., Sparatore, B., Salamino, F., Michetti, M., Sacco, O. &Horecker, B. L. (1985) Role of phospholipids in the activation of the Ca2+ -dependent neutral proteinase of human erythrocytes.Biochem. Biophys. Res. Commun. 129, 389–95.
Reddy, M. K., Etlinger, J. D., Rabinowitz, M., Fischman, D. A. &Zak, R. (1975) Removal of Z-lines and alpha-actinin from isolated myofibrils by a calcium-activated neutral protease.J. biol. Chem. 250, 4278–84.
Reedy, M. K., Leonard, K. R., Freeman, R &Arad, T. (1981) Thick myofilament mass determination by electron scattering measurements with the scanning transmission electron microscope.J. Musc. Res. Cell Motility 2, 45–64.
Saide, J. D. &Ullrick, W. C. (1974) Purification and properties of the isolated honeybee Z-disc.J. molec. Biol. 87, 671–83.
Sainsbury, G. M. &Bullard, B. (1980) New proline-rich proteins in isolated insect Z-discs.Biochem. J. 191, 333–9.
Sainsbury, G. M. &Hulmes, D. (1977) Notes on the structure of the Z-disc of insect flight muscle. InInsect Flight Muscle (edited byTregear, R. T.), pp. 75–8. Amsterdam: North-Holland.
Starkey, P. M. &Barrett, A. J. (1976) Neutral proteinases of human spleen. Purification and criteria for homogeneity of elastase and cathepsin G.Biochem. J. 155, 255–63.
Sugita, H., Ishiura, S., Suzuki, K. &Imahori, K. (1980) Ca2+ -activated neutral protease and its inhibitors:in vitro effect on intact myofibrils.Muscle Nerve 3, 335–9.
Suzuki, K. (1987) Calcium-activated neutral protease: domain structure and activity regulation.Trends in biochem. Sci. 12, 103–5.
Suzuki, A., Nonami, Y., &Goll, D. E. (1975) Proteins released from myofibrils by CASF (Ca2+-activated sarcoplasmic factor) and trypsin.Agric. Biol. Chem,39, 1461–7.
Szpacenko, A., Kay, J., Goll, D. E. &Otsuka, Y. (1981) A different form of the Ca2+ -dependent proteinase activated by micromolar levels of Ca2+. InProteinases and their Inhibitors. Structure, Function and Applied Aspects. (edited byTurk, V. &Vitale, Lj.), pp. 151–61. Ljubljana, Oxford: Mladinska knjiga-Pergamon Press.
Turner, P. R., Westwood, T., Regan, C. M. &Steinhardt, R. A. (1988) Increased protein degradation results from elevated free calcium levels found in muscle frommdx mice.Nature 335, 735–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bullard, B., Sainsbury, G. & Miller, N. Digestion of proteins associated with the Z-disc by calpain. J Muscle Res Cell Motil 11, 271–279 (1990). https://doi.org/10.1007/BF01843580
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01843580