Abstract
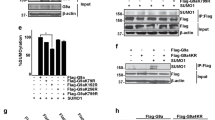
Regulatory transcription factors of the Pax family play fundamental roles in the function of multipotent cells during vertebrate development, post-natal regeneration, and cancer. Pax7 and its homologue Pax3 are important players in neural crest and muscle development. Both genes are coexpressed in various tissues and are thought to provide similar, but not identical, functions. The mechanisms that allow specific regulation of Pax7 remain largely unknown. Here, we report for the first time that Pax7 is regulated by SUMOylation. We identify the interaction of Pax7 with Ubc9, the SUMO conjugating enzyme, and reveal that SUMOylation machinery is enriched in neural crest precursors and plays a critical role in NC development. We demonstrate that Pax7 becomes SUMOylated and identify an essential role for lysine 85 (K85) in Pax7-SUMOylation. Despite high conservation surrounding K85 amongst Pax genes, we were unable to identify SUMOylation of other Pax proteins tested, including Pax3. Using a non-SUMOylatable Pax7 variant (K85 X R), we demonstrate that SUMOylation is essential for the function of Pax7 in neural crest development, C2C12 myogenic differentiation, and transcriptional transactivation. Our study provides new mechanistic insight into the molecular regulation of Pax7’s function by SUMOylation in neural crest and muscle development.







Similar content being viewed by others
References
Nusslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287:795–801
Chi N, Epstein JA (2002) Getting your Pax straight: Pax proteins in development and disease. Trends Genet 18:41–47
Robson EJ, He SJ, Eccles MR (2006) A PANorama of PAX genes in cancer and development. Nat Rev Cancer 6:52–62
Wang Q et al (2008) Pax genes in embryogenesis and oncogenesis. J Cell Mol Med 12:2281–2294
Bopp D, Burri M, Baumgartner S, Frigerio G, Noll M (1986) Conservation of a large protein domain in the segmentation gene paired and in functionally related genes of Drosophila. Cell 47:1033–1040
Treisman J, Harris E, Desplan C (1991) The paired box encodes a second DNA-binding domain in the paired homeo domain protein. Genes Dev 5:594–604
Czerny T, Schaffner G, Busslinger M (1993) DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev 7:2048–2061
Xu W, Rould MA, Jun S, Desplan C, Pabo CO (1995) Crystal structure of a paired domain-DNA complex at 2.5 A resolution reveals structural basis for Pax developmental mutations. Cell 80:639–650
Jun S, Desplan C (1996) Cooperative interactions between paired domain and homeodomain. Development 122:2639–2650
Mansouri A, Goudreau G, Gruss P (1999) Pax genes and their role in organogenesis. Cancer Res 59:1707s–1710s
Mansouri A, Stoykova A, Torres M, Gruss P (1996) Dysgenesis of cephalic neural crest derivatives in Pax7−/− mutant mice. Development 122:831–838
Basch ML, Bronner-Fraser M, Garcia-Castro MI (2006) Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 441:218–222
Seale P et al (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102:777–786
Seale P, Ishibashi J, Scime A, Rudnicki MA (2004) Pax7 is necessary and sufficient for the myogenic specification of CD45+: Sca1+ stem cells from injured muscle. PLoS Biol 2:E130
Relaix F et al (2006) Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol 172:91–102
Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA (2006) Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol 172:103–113
Olguin HC, Yang Z, Tapscott SJ, Olwin BB (2007) Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol 177:769–779
Lepper C, Conway SJ, Fan CM (2009) Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460:627–631
Buckingham M, Relaix F (2007) The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol 23:645–673
Boutet SC, Disatnik MH, Chan LS, Iori K, Rando TA (2007) Regulation of Pax3 by proteasomal degradation of monoubiquitinated protein in skeletal muscle progenitors. Cell 130:349–362
Hollenbach AD, Sublett JE, McPherson CJ, Grosveld G (1999) The Pax3-FKHR oncoprotein is unresponsive to the Pax3-associated repressor hDaxx. EMBO J 18:3702–3711
Muramatsu T, Mizutani Y, Ohmori Y, Okumura J (1997) Comparison of three nonviral transfection methods for foreign gene expression in early chicken embryos in ovo. Biochem Biophys Res Commun 230:376–380
Chapman SC, Collignon J, Schoenwolf GC, Lumsden A (2001) Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dyn 220:284–289
Henrique D et al (1995) Expression of a Delta homologue in prospective neurons in the chick. Nature 375:787–790
Betters E, Liu Y, Kjaeldgaard A, Sundstrom E, Garcia-Castro MI (2010) Analysis of early human neural crest development. Dev Biol 344:578–592
Johnson ES, Blobel G (1997) Ubc9p is the conjugating enzyme for the ubiquitin-like protein Smt3p. J Biol Chem 272:26799–26802
Kerscher O, Felberbaum R, Hochstrasser M (2006) Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol 22:159–180
Gareau JR, Lima CD (2010) The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol 11:861–871
Suzuki T et al (1999) A new 30-kDa ubiquitin-related SUMO-1 hydrolase from bovine brain. J Biol Chem 274:31131–31134
Otto A, Schmidt C, Patel K (2006) Pax3 and Pax7 expression and regulation in the avian embryo. Anat Embryol (Berl) 211:293–310
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Nowak M, Hammerschmidt M (2006) Ubc9 regulates mitosis and cell survival during zebrafish development. Mol Biol Cell 17:5324–5336
Alkuraya FS et al (2006) SUMO1 haploinsufficiency leads to cleft lip and palate. Science 313:1751
Song T et al (2008) SUMO1 polymorphisms are associated with non-syndromic cleft lip with or without cleft palate. Biochem Biophys Res Commun 377:1265–1268
Carter TC et al (2010) Testing reported associations of genetic risk factors for oral clefts in a large Irish study population. Birth Defects Res A Clin Mol Teratol 88:84–93
Jia ZL, Shi B, Xu X, Kong XL (2011) Interactions between small ubiquitin-like modifier 1 and nonsyndromic orofacial clefts. DNA Cell Biol 30:235–240
Evdokimov E, Sharma P, Lockett SJ, Lualdi M, Kuehn MR (2008) Loss of SUMO1 in mice affects RanGAP1 localization and formation of PML nuclear bodies, but is not lethal as it can be compensated by SUMO2 or SUMO3. J Cell Sci 121:4106–4113
Zhang FP et al (2008) Sumo-1 function is dispensable in normal mouse development. Mol Cell Biol 28:5381–5390
Wang J et al (2011) Defective sumoylation pathway directs congenital heart disease. Birth Defects Res A Clin Mol Teratol 91:468–476
Rodriguez MS, Dargemont C, Hay RT (2001) SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J Biol Chem 276:12654–12659
Ren J et al (2009) Systematic study of protein SUMOylation: development of a site-specific predictor of SUMOsp 2.0. Proteomics 9:3409–3412
Halevy O et al (2004) Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn 231:489–502
Oustanina S, Hause G, Braun T (2004) Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J 23:3430–3439
Olguin HC, Olwin BB (2004) Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol 275:375–388
Zammit PS et al (2006) Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci 119:1824–1832
McKinnell IW et al (2008) Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol 10:77–84
McFarlane C et al (2008) Myostatin signals through Pax7 to regulate satellite cell self-renewal. Exp Cell Res 314:317–329
Kumar D, Shadrach JL, Wagers AJ, Lassar AB (2009) Id3 is a direct transcriptional target of Pax7 in quiescent satellite cells. Mol Biol Cell 20:3170–3177
Collins CA et al (2009) Integrated functions of Pax3 and Pax7 in the regulation of proliferation, cell size and myogenic differentiation. PLoS ONE 4:e4475
Murdoch B, DelConte C, Garcia-Castro MI (2012) Pax7 lineage contributions to the mammalian neural crest. PLoS One 7(7):e41089. doi:10.1371/journal.phone.0041089
Murdoch B, DelConte C, Garcia-Castro MI (2010) Embryonic Pax7-expressing progenitors contribute multiple cell types to the postnatal olfactory epithelium. J Neurosci 30:9523–9532
Olguin HC, Pisconti A (2011) Marking the tempo for myogenesis: Pax7 and the regulation of muscle stem cell fate decisions. J Cell Mol Med
Liem KF Jr, Tremml G, Roelink H, Jessell TM (1995) Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 82:969–979
Linker C et al (2009) Cell communication with the neural plate is required for induction of neural markers by BMP inhibition: evidence for homeogenetic induction and implications for Xenopus animal cap and chick explant assays. Dev Biol 327:478–486
Maczkowiak F et al (2010) The Pax3 and Pax7 paralogs cooperate in neural and neural crest patterning using distinct molecular mechanisms, in Xenopus laevis embryos. Dev Biol 340:381–396
Stuhlmiller TJ, Garcia-Castro MI (2012) FGF/MAPK signaling is required in the gastrula epiblast for avian neural crest induction. Development 139:289–300
Taylor KM, Labonne C (2005) SoxE factors function equivalently during neural crest and inner ear development and their activity is regulated by SUMOylation. Dev Cell 9:593–603
de Cristofaro T et al (2009) Pax8 protein stability is controlled by sumoylation. J Mol Endocrinol 42:35–46
Yan Q et al (2010) Sumoylation activates the transcriptional activity of Pax-6, an important transcription factor for eye and brain development. Proc Natl Acad Sci USA 107:21034–21039
Acknowledgments
This work was funded by NIH RO1DE017914. We thank members of the García-Castro laboratory for comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luan, Z., Liu, Y., Stuhlmiller, T.J. et al. SUMOylation of Pax7 is essential for neural crest and muscle development. Cell. Mol. Life Sci. 70, 1793–1806 (2013). https://doi.org/10.1007/s00018-012-1220-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1220-1