Abstract
Hyperpolarization-activated cyclic nucleotide-gated 1 (HCN1) channels carry Ih, which contributes to neuronal excitability and signal transmission in the nervous system. Controlling the trafficking of HCN1 is an important aspect of its regulation, yet the details of this process are poorly understood. Here, we investigated how the C-terminus of HCN1 regulates trafficking by testing for its ability to redirect the localization of a non-targeted reporter in transgenic Xenopus laevis photoreceptors. We found that HCN1 contains an ER localization signal and through a series of deletion constructs, identified the responsible di-arginine ER retention signal. This signal is located in the intrinsically disordered region of the C-terminus of HCN1. To test the function of the ER retention signal in intact channels, we expressed wild type and mutant HCN1 in HEK293 cells and found this signal negatively regulates surface expression of HCN1. In summary, we report a new mode of regulating HCN1 trafficking: through the use of a di-arginine ER retention signal that monitors processing of the channel in the early secretory pathway.
Similar content being viewed by others
Introduction
Hyperpolarization-activated cyclic nucleotide-gated channels (HCN) are expressed in the brain and heart where they can modulate signal integration, synaptic transmission, or rhythmic changes in membrane excitability [1]. They are members of the voltage gated potassium channel superfamily and consist of four proteins, HCN1, HCN2, HCN3 and HCN4, that form heterotetramic or homotetrameric channels in a tissue-specific manner. The HCN channels are activated by hyperpolarization, and further modulated by cAMP. Upon activation, they carry inward currents consisting of K+ and Na+ [2]. Not surprisingly, disruption of their activity influences behavior and is pathological. For instance, alterations in HCN2 and HCN4 lead to cardiac arrhythmia [3]. HCN1 is more broadly expressed and influences behavior in diverse ways; it contributes to vision at medium and bright light intensities [4–6], memory formation [7], and sleep [8]. Perturbation of HCN1 underlies some forms of epilepsy and chronic pain [9–11]. The ongoing efforts to elucidate the mechanisms of HCN channel regulation are critical to making progress in understanding their roles in disease progression.
HCN channels are regulated by several convergent mechanisms. HCN channels open relatively slowly in response to membrane hyperpolarization and their gating is modulated by factors including cAMP, phosphatidylinositol 4,5-bisphosphate (PIP2), and the accessory subunit, tetratricopeptide repeat-containing Rab8b interacting protein (TRIP8b) [1]. Controlling subcellular trafficking is another important aspect of channel regulation but is not well understood. This is in part because there are likely overlapping mechanisms to account for the range of HCN localization found across cells.
HCN1 can be found broadly distributed throughout the plasma membrane of a neuron or be restricted to a particular compartment. In photoreceptors, only HCN1 is expressed; it is most abundant in inner segments and excluded from outer segments [5, 12–14]. In cochlear hair cells, HCN1 is localized to stereocilia [15, 16]. In hippocampal neurons, HCN1 is strikingly concentrated in distal dendrites [17]. In such neurons where HCN1 is concentrated in the distal dendrites, TRIP8b is the major regulator of HCN1 trafficking as it is essential for reducing axonal, but enhancing, dendritic localization of the channel [10, 18–21]. However, TRIP8b is not required to localize HCN1 to the plasma membrane of pre-synaptic terminals or retinal neurons [22, 23]. Determining how the localization of HCN channels in various cells and compartments is controlled is a current challenge for the field.
The cytoplasmic C-terminus of HCN1 and related channels is important for trafficking. Their C-termini contains two conserved structured regions, the C-linker which contributes to tetramerization and the cyclic nucleotide-binding domain (CNBD) which allows for modulation by cAMP, followed by a non-conserved region [24]. The non-conserved region contains the binding sites for TRIP8b [18, 20]. It also contains a binding site for filamin-A, which has been shown to promote clustering of the channel in the plasma membrane [25]. It is via the C-terminus that HCN1 and HCN2 bind KCNE2, a promiscuous potassium channel accessory subunit that increases the surface expression of its partners [26–29]. HCN3 surface expression is enhanced by its own accessory subunit, K+ channel tetramerization domain-containing protein 3 (KCTD3), which interacts with the portion of the C-terminus containing the C-linker and CNBD domains [30]. In a more distantly related Shaker-like K+ channel, Arabidopsis thaliana 2, specific residues in the C-linker are necessary for promoting surface expression of the channel [31]. It is not known how these factors influence HCN1 trafficking in different types of specialized cells such as photoreceptors.
Our purpose in this study was to identify additional regulatory elements controlling the trafficking of HCN1. We used Xenopus laevis photoreceptors, a powerful model system for dissecting neuronal and ciliary trafficking pathways [32]. Fusing the C-terminus of HCN1 to a reporter that alone accumulates in the modified primary cilia resulted in redirecting the reporter to the ER within the inner segment. By analyzing the localization of a series of mutants of this reporter-HCN1 CT construct, we uncovered a di-arginine ER retention motif. Di-arginine motifs have been found in a select subset of channels, and receptors and have been proposed to function as signals for improperly assembled channels. This predicts that disruption of the di-arginine motif in otherwise intact HCN1 channels would increase the amount of channel that exits the ER and localizes to the cell surface. Immunocytochemistry and biotinylation assays of HCN1 channels expressed in HEK293 cells verified that mutation of the di-arginine motif caused an increase in surface expression. In conclusion, a di-arginine ER retention signal that influences the trafficking of HCN1 from the ER to the plasma membrane has been discovered. We propose that this signal works in combination with as yet to be determined forward trafficking signals to ensure the proper delivery of functional HCN1 channels to the plasma membrane of neurons.
Materials and methods
Animals
Xenopus laevis were purchased from Nasco (Fort Atkinson, WI USA) and maintained by the Office of Animal Research at the University of Iowa. All experiments were approved by the Institutional Animal Care and Use Committee and adhered to the ARVO guidelines for animal use in vision research. Transgenic Xenopus tadpoles were generated using restriction enzyme-mediated integration as previously described [33, 34]). Briefly, linearized and purified plasmid DNA was integrated into sperm nuclei in the presence of a Ca2+-activated egg cytoplasmic extract. Treated nuclei were transplanted into unfertilized eggs obtained by inducing mature animals with human chorionic gonadotropin (Prospec, Ness-Ziona, Israel). Embryos were housed in 0.3× Marc’s Modified Ringer (30 mM NaCl, 0.6 mM KCL, 0.3 mM MgCl2, 0.6 mM CaCl2, 1.5 mM HEPES, pH 7.4). Transgenic tadpoles were identified at St 42 by screening for GFP expression in the eye and humanely euthanized between St 45 and 55 by immersion in 0.2 % tricaine (Sigma-Aldrich, St. Louis, MO, USA), prior to processing for immunohistochemistry.
Molecular cloning
All constructs used for transgenesis were subcloned into the XOP5.5 vector containing the Xenopus opsin promoter to ensure rod photoreceptor-specific expression [35]. All inserts were generated by standard PCR protocols and verified by Sanger sequencing (Iowa Institute of Human Genetics, University of Iowa, Iowa, USA). The membrane reporter consists of the transmembrane domain from mouse activin receptor type 2A (aa 117–165), followed by EGFP, followed by a palmitoylated peptide from Xenopus rhodopsin (aa 311–349). The template for the X. tropicalis HCN1 inserts was obtained from retina cDNA; amino acid numbers thereby correspond to accession #XP002933077. The sequences of the primers used are provided in Supplemental Fig. 1. All constructs used for transfection of HEK293 cells were subcloned into the pEGFPN1 vector containing the CMV promoter. The mouse HCN1 insert was a generous gift from Dr. Yoav Noam [36, 37], the GFP-TRIP8b (1a-4) insert was a generous gift from Dr. Dane Chetkovich [36].
Immunostaining
Transgenic tadpoles were fixed in 4 % paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA), cryoprotected in 30 % sucrose, and frozen in Tissue-Tek O.C.T (Electron Microscopy Sciences, Hatfield, PA, USA). Sections collected on charged glass slides were permeabilized in 0.5 % Triton X-100, blocked with 5 % goat serum and 0.5 % Triton X-100 in PBS, incubated in mouse α-GFP antibodies (diluted 1:500; Clontech Laboratories, Mountain View, CA, USA) or rabbit α-calnexin antibodies (diluted 1:100; Enzo Life Sciences, Farmingdale, NY USA), followed by secondary goat α-rabbit or goat α-mouse antibodies conjugated to Alexa 488 or 568 (Life Technologies, Grand Island, NY, USA) mixed with 2 μg/mL Hoechst 33342 (Life Technologies, Grand Island, NY, USA) to label the nuclei. Images were collected using a Zeiss 710 confocal microscope (Central Microscopy Research Facility, University of Iowa, IA, USA). Manipulation of images was limited to adjusting the brightness and contrast levels using Zen Light 2009 (Carl Zeiss Microscopy, Jena, Germany) or Photoshop (Adobe Systems Inc., San Jose, CA, USA). A minimum of four individual transgenic tadpoles were studied for every DNA construct.
Cell culture
HEK293 cells (ATCC, Manassas, VA, USA) were maintained in DMEM supplemented with 10 % FBS, 250 μg/mL fungizone and 1 % penicillin/streptomycin (Life Technologies, Grand Island, NY, USA). For immunocytochemistry, cells were seeded at a density of 0.3 × 106 cells/mL on chambered glass coverslips (Thermo Fisher Scientific Inc. Waltham, MA, USA,). A total of 0.4 μg plasmid DNA (0.2 μg for the HA-HCN1 constructs and 0.2 μg for the GFP or GFP-TRIP8b) was transfected using Lipofectamine 2000 (Life Technologies, Grand Island, NY, USA). 24 h post-transfection, cells were fixed in 4 % paraformaldehyde and stained as described above with α-HA antibodies (diluted 1:500; Thermo Scientific, Waltham, MA, USA). Each experiment was replicated a minimum of three times.
Biotinylation assays
HEK293 cells were seeded at a density of 0.75 × 106 cells/mL in 6-well plates and transfected with a total of 2 μg plasmid DNA (1 μg for the HA-HCN1 constructs and 1 μg for the GFP or GFP-TRIP8b) using Lipofectamine 2000 (Life Technologies, Grand Island, NY, USA). 24 h post-transfection, cells were incubated with 1 mg/mL sulfo-NHS-SS-biotin (Thermo Scientific, Waltham, MA, USA) for 15 min at 4 °C to label only the surface proteins. The reaction was quenched in TBS (50 mM Tris–Cl and 150 mM NaCl, pH 7.5) for 15 min at 4 °C, and washed in PBS. The biotinylated cells were homogenized in 200 µL lysis buffer (50 mM Tris–HCl, 10 mM NaCl, 0.32 M sucrose, 5 mM EDTA, 2.5 mM EGTA, 1.5 % Triton X-100, 0.75 % DOC and 0.1 % SDS, pH 7.4) supplemented with protease inhibitor cocktail (Complete, mini, Roche, Basel, Switzerland). Insoluble material was removed by centrifugation at 16,000×g for 10 min. 100 µL of the supernatant was collected and incubated with 50 µL NeutrAvidin agarose resin (Thermo Scientific Inc., Waltham, MA, USA,), with rotation at 4 °C overnight. Proteins bound to the beads were washed with PBS three times and eluted with 50 µL reducing NuPage LDS sample buffer (Life Technologies, Grand Island, NY, USA) followed by Western blotting with the following antibodies: rabbit α-HCN1 [23], rabbit α-TRIP8b [23], mouse α-NKA (M7-PB-E9, diluted 1:1,000; Santa Cruz Biotechnology, Dallas, TX, USA), rabbit α-GAPDH (diluted 1:500; Abcam, Cambridge, England), goat α-rabbit HRP and goat α-mouse HRP (Sigma-Aldrich, St. Louis, MO, USA). The software package Image Studio v3.1 (LI-COR Biosciences, Lincoln, NE, USA) was used for analysis of the blots. Each experiment was replicated a minimum of three times.
Results
The C-terminus of HCN1 redirects an integral membrane reporter to the ER in Xenopus photoreceptors
Photoreceptors express homotetrameric HCN1 channels. The major features of each monomer are an intrinsically disordered cytoplasmic N-terminus, followed by six transmembrane domains, and a cytoplasmic C-terminus. The membrane proximal portion of the C-terminus consists of the C-linker and cyclic nucleotide-binding domain (CNBD) followed by a long intrinsically disordered region (Fig. 1a–b) [1, 38]. To determine the contribution of the HCN1 C-terminus to trafficking of the channel in photoreceptors, we fused this sequence to a membrane reporter. This reporter consists of a single transmembrane domain anchoring GFP, which is followed by a peptide sequence that can be dually palmitoylated (Fig. 1c) [39]. The advantage of this reporter is that it accumulates in the outer segments when expressed in transgenic Xenopus photoreceptors (Fig. 2a), but relocalizes when fused to a protein sequence containing targeting information. Furthermore, as an integral membrane protein it will be co-translationally inserted into the ER, mimicking the initial steps of HCN1 trafficking. The membrane reporter fused to the C-terminus of X. tropicalis HCN1 (Figs. 1c–d, 2b) was found in internal compartments within the inner segment. This pattern is reminiscent of the distribution of ER in frog rod photoreceptors [40]. Immunostaining the same section with antibodies against calnexin (an ER resident protein) verified co-localization of the exogenous proteins with the ER (Fig. 2b–d). This suggested that the C-terminus of HCN1 contains an ER-targeting signal; a possibility tested with the series of deletion mutants described below and summarized in Fig. 1d.
Overview of HCN1 structure and experimental design. a Schematic representation of an HCN1 homotetramer (left). Each monomer (cyan) consists of six transmembrane domains with cytoplasmic N- and C-termini (right). The cyan oval in the C-terminus indicates the relative position of the CNBD. b Disorder analysis of HCN1 using meta protein disorder prediction system. Residues above the threshold (gray dashed line) are predicted to be intrinsically disordered. c A cartoon of the reporter (left) consisting of a single pass transmembrane domain (red), GFP (green) and a palmitoylated peptide (red) to which the C-terminus (cyan) of Xenopus HCN1 (or portions thereof) was attached. d A summary of the design and targeting behavior of all constructs expressed in transgenic Xenopus photoreceptors. The linear arrangement of sequence motifs in the HCN1 C-terminus is displayed on top. The range of HCN1 amino acids attached to the reporter in each construct is listed in the first column. The gray shaded area is required for ER localization. TMD transmembrane domain, CNBD cyclic nucleotide-binding domain, QP glutamine- and proline-rich region, OS outer segment, ER endoplasmic reticulum
The C-terminus of HCN1 directs localization to the ER. a Transgenic Xenopus rod photoreceptors expressing the reporter alone or b in fusion to the cytoplasmic C-terminus (aa 373–839) of Xenopus HCN1. The transgenically expressed protein (b, green) colocalizes with calnexin (c, red) in the inner segment (d, transmitted light). OS outer segments, IS inner segments, N (blue) nuclei, ST synaptic terminals. Scale bars 5 μm
The ER-targeting signal is located within the intrinsically disordered portion of the C-terminus
We first tested if the C-linker or/and CNBD are responsible for the ER localization of the reporter-HCN1 CT construct. Note, the expression of fusion proteins containing the CNBD resulted in large numbers of intracellular aggregates and sometimes cell death in transgenic photoreceptors (data not shown). Incorporation of a single point mutation, R524E, previously shown to disrupt cyclic nucleotide binding but not the other functions of HCN1 [41], significantly reduced this problem. Therefore, the five constructs tested in Xenopus photoreceptors that contain the CNBD include the R524E modification. Using either the individual C-linker or CNBD domains fused to the reporter, resulted in proteins that were not localized to either the outer segment or ER.
Instead, they were found accumulating in large amorphous structures in the apical portion of the inner segments and/or synaptic terminals (Fig. 3a–b). These structures may be extremely large aggregates or the isolation of these two structural domains may have exposed a cryptic association with mitochondria. Regardless, the problem was abrogated when the C-linker and CNBD were used in tandem. This caused the reporter to localize in outer segments (Fig. 3c). Appending the distal C-terminus to the CNBD or using just the distal C-terminus restored ER localization of the reporter (Fig. 3d–e). Together these data indicate that the ER-targeting signal is located downstream of the structured domains.
The C-linker and CNBD are not required for ER localization. Transgenic Xenopus rod photoreceptors expressing the indicated Reporter-HCN1 fragments detailed in Fig. 1d. OS outer segments, IS inner segments, N (blue) nuclei, ST synaptic terminals. Scale bars 5 μm
We next tested if any readily recognizable motifs in the distal C-terminus of HCN1 were responsible for the ER localization. Two regions drew our attention. There is a stretch of amino acids enriched in glutamines and prolines, that we call the QP region. The function of this region is currently unknown. Also, the last three amino acids (SNL) are highly conserved in HCN channels and serve as the binding site for the accessory subunit, TRIP8b [18, 20, 42]. Deleting the SNL signal did not prevent the ER localization (Fig. 4a), consistent with our previous finding that TRIP8b is not required for regulating HCN1 trafficking in photoreceptors [23]. Similarly, eliminating the QP region did not alter the ER localization pattern (Fig. 4b).
Identification of a di-arginine ER retention signal. a–d Transgenic Xenopus rod photoreceptors expressing the indicated Reporter-HCN1 fragments detailed in Fig. 1d. OS outer segments, IS inner segments, N (blue) nuclei, ST synaptic terminals. Scale bars 5 μm. e ClustalW sequence alignment of X. tropicalis HCN1 (aa 576–635) to HCN1 from various animal species. Identical residues shaded in black, partially conserved residues in gray, with the di-arginine motif outlined in red [asterisks mark arginines forming the RxR (red) or RxRxR (blue) motif]. Accession numbers are: X. tropicalis, XP002933077; X. laevis a, b deduced from genomic scaffold v7.1 52441 and 337825. C. mydas, XP_007052900; A. sinensis, XP_006017356; C. livia, XP_005500951; F. peregrinus, XP_005242028; G. gallus, XP429145; B. mutus, ELR46479; R. norvegicus, W9JKB0; M. musculus, O88704; H. sapiens, O60741
Comparison of all the constructs exhibiting ER localization tested thus far allowed us to constrain the region of interest to a shared 60 amino acid long region in the beginning of the disordered portion of the C-terminus (Fig. 1d). Deletion of this region resulted in localization of the reporter to outer segments (Fig. 4c, also Fig. 3c). Considering that motifs involved in protein targeting are often conserved across species, we examined an alignment of this region which drew our attention to a putative RxR di-arginine ER retention signal [43]. This motif is conserved among amphibians, reptiles, birds, and mammals (Fig. 4e). The sequence of HCN1 in X. tropicalis varies slightly from the other species in having two overlapping RxR motifs (RMRTR). Three point mutations (R631A, R633A, R635A) were made to test if either motif was functional. Indeed, this resulted in localization of the reporter to the outer segments (Fig. 4d, compare with Fig. 3e). In conclusion, a conserved di-arginine ER retention signal in the middle of the HCN1 C-terminus was responsible for the redistribution of the membrane reporter to the internal membranes of the inner segment.
The HCN1 di-arginine ER retention signal regulates the amount of channel trafficked to the cell surface
Di-arginine motifs found in a number of channels and receptors are thought to mediate constitutive ER retention of partially folded or unassembled oligomers. Proper assembly of the protein complex likely causes a physical masking of the di-arginine motif, thereby allowing exit from the ER [44]. The robust ER localization we observed in Xenopus photoreceptors is consistent with this idea as the reporter constructs carrying only the C-terminus of HCN1 cannot assemble into a properly organized channel. To substantiate this, the requirement for the di-arginine motif should be tested in the context of the full-length HCN1. This is problematic in transgenic photoreceptors because the experimental versions of HCN1 would likely oligomerize with endogenous channels and override any mutations we incorporate. Therefore, we turned to a simpler model system for assessing the efficiency of ER exit for HCN1 channels.
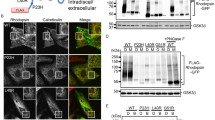
We expressed HA-tagged mouse HCN1WT or HCN1MUT (R648A and R650A, thus mutating the RxR motif to AxA) in HEK293 cells. Insertion of the HA tag in the second extracellular loop of mammalian HCN1 has been demonstrated to result in functional channels with normal biophysical properties when overexpressed in HEK293 cells [36, 37]. Immunostaining of transfected cells revealed that HCN1WT was largely localized to the ER (Supplemental Fig. 2). However, plasma membrane localization of HCN1MUT was prominent compared to that of the wild-type channel (Fig. 5a–b).
The di-arginine motif influences plasma membrane localization of HCN1. Immunostaining of HEK293 cells expressing the following proteins: a wild-type HA-tagged HCN1 and GFP; b HA-tagged HCN1 with a mutated ER retention signal (RxR to AxA) and GFP; c wild-type HA-HCN1 and GFP-TRIP8b; d GFP alone. HA tag (red), GFP (green), Nuclei (blue), and scale bars 10 μm
As a positive control, HCN1WT was co-expressed with GFP tagged TRIP8b (1a-4), because this specific splice isoform of TRIP8b is known to increase surface expression of HCN1 [19, 21, 45]. Indeed the plasma membrane localization of HCN1WT was dramatically increased (Fig. 5c). Note, in experiments lacking GFP-TRIP8b (1a-4), co-transfection of GFP was used to balance the amount of DNA transfected. In the negative control, only GFP was expressed to demonstrate specific HA immunostaining. The amount of plasma membrane HCN1 appeared greatest for HCN1WT + TRIP8b, intermediate for HCN1MUT, and lowest for HCN1WT alone.
To quantify the surface expression of HCN1 we used biotinylation assays. Briefly, all proteins presented on the cell surface were labeled with a membrane-impermeable biotin reagent. The biotinylated surface proteins were subsequently isolated by an affinity column, and Western blotting was used to compare the amount of HCN1 in the pool of surface proteins versus total cellular proteins. Co-transfection of GFP-TRIP8b (1a-4) with HCN1WT increased the surface expression of HCN1WT by fivefold. Consistent with the qualitative imaging analysis, HCN1MUT also increased surface expression over HCN1WT but only by twofold (Fig. 6). In conclusion, these data demonstrate that the di-arginine motif functions in the intact channel by limiting the amount of channel allowed to traffic from the ER to the plasma membrane.
Mutating the di-arginine motif enhances the surface expression. Biotinylation assays of HEK293 cells transfected as described in (a, Fig. 5). a After biotinylation of transfected cells, surface proteins were pulled down using NeutrAvidin beads. The level of HCN1 and TRIP8b in the total and surface pools was detected by Western blotting. NKA and GAPDH were used as controls for membrane and cytosolic proteins, respectively. b Densitometry of Western blots represented in (a) was used to calculate the surface to total ratio of HCN1 after normalization to the loading control NKA. Asterisks indicate statistical significance with p < 0.01 (t test)
Discussion
The central finding of this study is that HCN1 contains a di-arginine ER retention signal, conserved across species, that influences the amount of channel trafficked to the plasma membrane of both undifferentiated and polarized cells. As one of the carriers of Ih, HCN1 plays essential roles in neuronal cells by modulating membrane excitability and signal integration thereby influencing a diverse set of processes. Controlling the amount of HCN1 available in the plasma membrane has a direct effect on the amount of current that can be conducted in response to various stimuli.
To date, a number of missense mutations reported in human HCN1 demonstrate that disrupting this channel leads to various forms of epilepsy [46, 47]. Most of these mutations occur near the pore, thus causing altered activity and concomitantly reducing expression of the channel. In places such as the retina, it seems that excess HCN1 is available as the 40 % reduction in HCN1 heterozygous mice or TRIP8b knockout mice does not grossly affect visual function [23]. This implies that an additional insult that further reduces the amount of surface expressed channel or a mutation in the di-arginine motif that would enhance the surface expression of a channel with altered activity could tip the balance and cause even more severe disorders.
The di-arginine ER retention signal is found in unrelated channels and receptors including NMDA receptors, GABAB receptors and ATP-sensitive potassium channels (KATP) [48–52]. It has been proposed that di-arginine motifs are only functional if found within approximately 16–46 Å from the membrane [53]. The di-arginine motif in HCN1 is separated from the membrane by the C-linker and CNBD that together occupy 52 Å in the crystal structure of HCN2 [24]. This does not strike us as significantly different and predict that as more structures for membrane proteins become available, other di-arginine motifs will be found within this range.
The mechanism through which di-arginine motif-containing proteins are retained in or retrieved to the ER is not well understood. We favor the hypothesis that exposure of the di-arginine motif allows for weak or transient interactions with as yet uncharacterized ER proteins and that subunit assembly masks the di-arginine motif, thus allowing escape from the ER. This is the model proposed for the ATP-sensitive potassium (KATP) channels, which require four inward rectifier potassium (Kir) channel subunits and four sulphonylurea receptor (SUR) subunits for proper assembly. Both Kir and SUR contain the di-arginine ER retention signal, and neither subunit is expressed at the plasma membrane alone. Assembly into a heterooctamer likely sterically hinders the di-arginine motifs, allowing trafficking to the plasma membrane [48].
Could the di-arginine motif in HCN1 function by a similar mechanism? We observed that fragments of HCN1 containing the di-arginine motif but incapable of forming a channel show robust ER localization. However, in intact channels, a mixture of plasma membrane and ER localized channels was observed with an intact di-arginine motif shifting the balance more toward the ER. This supports the idea that the di-arginine motif in HCN1 could function similar to the KATP channels. The only known subunit that heterooligomerizes with HCN1 in photoreceptors is TRIP8b, which we have shown is dispensable for surface expression of HCN1 in the retina [23]. Therefore, the di-arginine ER retention signal present in HCN1 is more likely monitoring homooligomerization in photoreceptors rather than heterooligomerization.
Specific HCN channels are enriched in particular brain regions although most show some degree of overlap such as hippocampal neurons, which express both HCN1 and HCN2 [54–58]. It is speculated that in these neurons HCN1 and HCN2 are forming heteromeric channels, which in model expression systems have distinct biophysical properties. Since HCN2 and HCN4 lack a di-arginine motif heterooligomerization with HCN1 would provide an additional level of control to the trafficking of these h-channel heterooligomers. On the other hand, HCN3 contains a putative di-arginine motif (RKR) sequence following the CNBD and interestingly, HCN3 has been shown to localize primarily to ER-like structures when transfected in opossum kidney cells [59]. Clearly, the di-arginine motif in HCN1 regulates trafficking out the endomembrane system, but the role of this motif in the trafficking of related channels remains to be dissected.
An alternative mechanism for the action of the di-arginine motif would be for it to allosterically respond to post-translational modifications signaling maturation of the channel, such as glycosylation or phosphorylation. HCN1 is N-glycosylated in the third extracellular loop. This modification is seen as a 15 kDa shift in the mobility of HCN1 in Western blots [54, 56]. As expected, biotinylation assays have shown an increase in the amount of the glycosylated form in the surface pool, from roughly 10 % of the total HCN1 to nearly half. [23]. The amount of unmodified HCN1 at the surface indicates that if glycosylation is a maturation signal it may be needed on only one or two subunits of the intact channel.
Tyrosine phosphorylation of the C-linker in HCN2 and HCN4 by Src kinase regulates channel gating [60, 61], but it is hard to imagine that this effect is functioning at the ER and influencing trafficking of these channels. Notably, the di-arginine motif is adjacent to a conserved tyrosine that is flanked by three to eight serines or tyrosines in an arrangement that in the mammalian, though not the Xenopus sequences, matches to the consensus phosphorylation sequences for several abundant kinases [43, 62, 63]. While experimental validation is required, it is tempting to think that phosphorylation at one or more of these sites contribute to the function of the di-arginine motif as in the case of the calcium-sensing receptor and NMDA receptor [51, 64].
Another outstanding question is how regulating trafficking in the early secretory pathway by the di-arginine motif is coordinated with other modulators of HCN1 trafficking. In this study, we focused on the role of the C-terminus exactly because this region has been found to contain the binding sites for proteins proposed to regulate HCN trafficking in various cells [1]. We had previously been surprised to find that loss of TRIP8b did not decrease the surface expression of HCN1 in the retina (beyond that dictated by the reduction in total HCN1 protein available) as it does in cortical neurons [23]. In this study that provided an advantage in that by using photoreceptors as one of our model systems we were able to reveal a previously undiscovered mode of regulation. When we mutated the di-arginine motif in our reporter-based assay, we observed that the reporter filled the outer segments but was not specifically accumulating in the plasma membrane of the inner segment, where the endogenous channel is found. This suggests one of two scenarios: either additional signals operating through the C-terminus of HCN1 can only function in the context of a full-length molecule and/or properly folded channel, or regions of HCN1 outside of the C-terminus carry signals that influence its trafficking. Future studies designed to distinguish among these possibilities would be valuable in mapping out the trafficking pathway of HCN1 from ER to plasma membrane which in turn should pinpoint targets that can be manipulated to modify the amounts of HCN1 that are trafficked to the surface in either healthy or diseased cells.
In summary, we identified a novel di-arginine ER retention signal in the C-terminus of HCN1. The signal functions to limit the amount of HCN1 that exits from the ER to the the plasma membrane. We propose this is part of the tight control imposed on HCN1 trafficking given that altered surface expression of HCN1 is often observed in diseases such as epilepsy [56].
Abbreviations
- HCN1:
-
Hyperpolarization and cyclic nucleotide-gated channel 1
- ER:
-
Endoplasmic reticulum
- TRIP8b:
-
Tetratricopeptide repeat-containing Rab8b interacting protein
- CNBD:
-
Cyclic nucleotide-binding domain
- KATP :
-
ATP-sensitive potassium channels
- Kir :
-
Inward rectifier potassium channel
- SUR:
-
Sulphonylurea receptor
References
Wahl-Schott C, Biel M (2009) HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci 66(3):470–494. doi:10.1007/s00018-008-8525-0
Pape HC (1996) Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58:299–327. doi:10.1146/annurev.ph.58.030196.001503
Roubille F, Tardif JC (2013) New therapeutic targets in cardiology: heart failure and arrhythmia: HCN channels. Circulation 127(19):1986–1996. doi:10.1161/CIRCULATIONAHA.112.000145
Barrow AJ, Wu SM (2009) Low-conductance HCN1 ion channels augment the frequency response of rod and cone photoreceptors. J Neurosci 29(18):5841–5853. doi:10.1523/JNEUROSCI.5746-08.2009
Knop GC, Seeliger MW, Thiel F, Mataruga A, Kaupp UB, Friedburg C, Tanimoto N, Muller F (2008) Light responses in the mouse retina are prolonged upon targeted deletion of the HCN1 channel gene. Eur J Neurosci 28(11):2221–2230. doi:10.1111/j.1460-9568.2008.06512.x
Seeliger MW, Brombas A, Weiler R, Humphries P, Knop G, Tanimoto N, Muller F (2011) Modulation of rod photoreceptor output by HCN1 channels is essential for regular mesopic cone vision. Nat Commun 2:532. doi:10.1038/ncomms1540
Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin D, Thompson RF, Siegelbaum SA, Kandel ER, Morozov A (2003) The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell 115(5):551–564
Kanyshkova T, Pawlowski M, Meuth P, Dube C, Bender RA, Brewster AL, Baumann A, Baram TZ, Pape HC, Budde T (2009) Postnatal expression pattern of HCN channel isoforms in thalamic neurons: relationship to maturation of thalamocortical oscillations. J Neurosci 29(27):8847–8857. doi:10.1523/JNEUROSCI.0689-09.2009
Noam Y, Bernard C, Baram TZ (2011) Towards an integrated view of HCN channel role in epilepsy. Curr Opin Neurobiol 21(6):873–879. doi:10.1016/j.conb.2011.06.013
Lewis AS, Vaidya SP, Blaiss CA, Liu Z, Stoub TR, Brager DH, Chen X, Bender RA, Estep CM, Popov AB, Kang CE, Van Veldhoven PP, Bayliss DA, Nicholson DA, Powell CM, Johnston D, Chetkovich DM (2011) Deletion of the hyperpolarization-activated cyclic nucleotide-gated channel auxiliary subunit TRIP8b impairs hippocampal Ih localization and function and promotes antidepressant behavior in mice. J Neurosci 31(20):7424–7440. doi:10.1523/JNEUROSCI.0936-11.2011
Lewis AS, Estep CM, Chetkovich DM (2010) The fast and slow ups and downs of HCN channel regulation. Channels (Austin) 4(3):215–231
Muller F, Scholten A, Ivanova E, Haverkamp S, Kremmer E, Kaupp UB (2003) HCN channels are expressed differentially in retinal bipolar cells and concentrated at synaptic terminals. Eur J Neurosci 17(10):2084–2096
Demontis GC, Moroni A, Gravante B, Altomare C, Longoni B, Cervetto L, DiFrancesco D (2002) Functional characterisation and subcellular localisation of HCN1 channels in rabbit retinal rod photoreceptors. J Physiol 542(Pt 1):89–97
Fyk-Kolodziej B, Pourcho RG (2007) Differential distribution of hyperpolarization-activated and cyclic nucleotide-gated channels in cone bipolar cells of the rat retina. J Comp Neurol 501(6):891–903. doi:10.1002/cne.21287
Ramakrishnan NA, Drescher MJ, Khan KM, Hatfield JS, Drescher DG (2012) HCN1 and HCN2 proteins are expressed in cochlear hair cells: HCN1 can form a ternary complex with protocadherin 15 CD3 and F-actin-binding filamin A or can interact with HCN2. J Biol Chem 287(45):37628–37646. doi:10.1074/jbc.M112.375832
Yi E, Roux I, Glowatzki E (2010) Dendritic HCN channels shape excitatory postsynaptic potentials at the inner hair cell afferent synapse in the mammalian cochlea. J Neurophysiol 103(5):2532–2543. doi:10.1152/jn.00506.2009
Santoro B, Wainger BJ, Siegelbaum SA (2004) Regulation of HCN channel surface expression by a novel C-terminal protein–protein interaction. J Neurosci 24(47):10750–10762. doi:10.1523/JNEUROSCI.3300-04.2004
Santoro B, Hu L, Liu H, Saponaro A, Pian P, Piskorowski RA, Moroni A, Siegelbaum SA (2011) TRIP8b regulates HCN1 channel trafficking and gating through two distinct C-terminal interaction sites. J Neurosci 31(11):4074–4086. doi:10.1523/JNEUROSCI.5707-10.2011
Piskorowski R, Santoro B, Siegelbaum SA (2011) TRIP8b splice forms act in concert to regulate the localization and expression of HCN1 channels in CA1 pyramidal neurons. Neuron 70(3):495–509. doi:10.1016/j.neuron.2011.03.023
Han Y, Noam Y, Lewis AS, Gallagher JJ, Wadman WJ, Baram TZ, Chetkovich DM (2011) Trafficking and gating of hyperpolarization-activated cyclic nucleotide-gated channels are regulated by interaction with tetratricopeptide repeat-containing Rab8b-interacting protein (TRIP8b) and cyclic AMP at distinct sites. J Biol Chem 286(23):20823–20834. doi:10.1074/jbc.M111.236125
Wilkars W, Liu Z, Lewis AS, Stoub TR, Ramos EM, Brandt N, Nicholson DA, Chetkovich DM, Bender RA (2012) Regulation of axonal HCN1 trafficking in perforant path involves expression of specific TRIP8b isoforms. PLoS ONE 7(2):e32181. doi:10.1371/journal.pone.0032181
Huang Z, Lujan R, Martinez-Hernandez J, Lewis AS, Chetkovich DM, Shah MM (2012) TRIP8b-independent trafficking and plasticity of adult cortical presynaptic HCN1 channels. J Neurosci 32(42):14835–14848. doi:10.1523/JNEUROSCI.1544-12.2012
Pan Y, Bhattarai S, Modestou M, Drack AV, Chetkovich DM, Baker SA (2014) TRIP8b is required for maximal expression of HCN1 in the mouse retina. PLoS ONE 9(1):e85850. doi:10.1371/journal.pone.0085850
Zagotta WN, Olivier NB, Black KD, Young EC, Olson R, Gouaux E (2003) Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 425(6954):200–205. doi:10.1038/nature01922
Gravante B, Barbuti A, Milanesi R, Zappi I, Viscomi C, DiFrancesco D (2004) Interaction of the pacemaker channel HCN1 with filamin A. J Biol Chem 279(42):43847–43853. doi:10.1074/jbc.M401598200
Eldstrom J, Fedida D (2011) The voltage-gated channel accessory protein KCNE2: multiple ion channel partners, multiple ways to long QT syndrome. Expert Rev Mol Med 13:e38. doi:10.1017/S1462399411002092
Qu J, Kryukova Y, Potapova IA, Doronin SV, Larsen M, Krishnamurthy G, Cohen IS, Robinson RB (2004) MiRP1 modulates HCN2 channel expression and gating in cardiac myocytes. J Biol Chem 279(42):43497–43502. doi:10.1074/jbc.M405018200
Yu H, Wu J, Potapova I, Wymore RT, Holmes B, Zuckerman J, Pan Z, Wang H, Shi W, Robinson RB, El-Maghrabi MR, Benjamin W, Dixon J, McKinnon D, Cohen IS, Wymore R (2001) MinK-related peptide 1: a beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ Res 88(12):E84–E87
Ying SW, Kanda VA, Hu Z, Purtell K, King EC, Abbott GW, Goldstein PA (2012) Targeted deletion of Kcne2 impairs HCN channel function in mouse thalamocortical circuits. PLoS ONE 7(8):e42756. doi:10.1371/journal.pone.0042756
Cao-Ehlker X, Zong X, Hammelmann V, Gruner C, Fenske S, Michalakis S, Wahl-Schott C, Biel M (2013) Up-regulation of hyperpolarization-activated cyclic nucleotide-gated channel 3 (HCN3) by specific interaction with K + channel tetramerization domain-containing protein 3 (KCTD3). J Biol Chem 288(11):7580–7589. doi:10.1074/jbc.M112.434803
Nieves-Cordones M, Chavanieu A, Jeanguenin L, Alcon C, Szponarski W, Estaran S, Cherel I, Zimmermann S, Sentenac H, Gaillard I (2014) Distinct amino acids in the C-linker domain of the Arabidopsis K + channel KAT2 determine its subcellular localization and activity at the plasma membrane. Plant Physiol 164(3):1415–1429. doi:10.1104/pp.113.229757
Burns ME, Arshavsky VY (2005) Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron 48(3):387–401. doi:10.1016/j.neuron.2005.10.014
Amaya E, Kroll KL (1999) A method for generating transgenic frog embryos. Methods Mol Biol 97:393–414
Kroll KL, Amaya E (1996) Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122(10):3173–3183
Knox BE, Schlueter C, Sanger BM, Green CB, Besharse JC (1998) Transgene expression in Xenopus rods. Febs Lett 423(2):117–121. doi:10.1016/S0014-5793(98)00018-0
Lewis AS, Schwartz E, Chan CS, Noam Y, Shin M, Wadman WJ, Surmeier DJ, Baram TZ, Macdonald RL, Chetkovich DM (2009) Alternatively spliced isoforms of TRIP8b differentially control h channel trafficking and function. J Neurosci 29(19):6250–6265. doi:10.1523/JNEUROSCI.0856-09.2009
Noam Y, Zha Q, Phan L, Wu RL, Chetkovich DM, Wadman WJ, Baram TZ (2010) Trafficking and surface expression of hyperpolarization-activated cyclic nucleotide-gated channels in hippocampal neurons. J Biol Chem 285(19):14724–14736. doi:10.1074/jbc.M109.070391
Ishida T, Kinoshita K (2008) Prediction of disordered regions in proteins based on the meta approach. Bioinformatics 24(11):1344–1348. doi:10.1093/bioinformatics/btn195
Tam BM, Moritz OL, Hurd LB, Papermaster DS (2000) Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J Cell Biol 151(7):1369–1380
Baker SA, Haeri M, Yoo P, Gospe SM 3rd, Skiba NP, Knox BE, Arshavsky VY (2008) The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J Cell Biol 183(3):485–498. doi:10.1083/jcb.200806009
Chen S, Wang J, Siegelbaum SA (2001) Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol 117(5):491–504
Bankston JR, Camp SS, DiMaio F, Lewis AS, Chetkovich DM, Zagotta WN (2012) Structure and stoichiometry of an accessory subunit TRIP8b interaction with hyperpolarization-activated cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 109(20):7899–7904. doi:10.1073/pnas.1201997109
Dinkel H, Van Roey K, Michael S, Davey NE, Weatheritt RJ, Born D, Speck T, Kruger D, Grebnev G, Kuban M, Strumillo M, Uyar B, Budd A, Altenberg B, Seiler M, Chemes LB, Glavina J, Sanchez IE, Diella F, Gibson TJ (2014) The eukaryotic linear motif resource ELM: 10 years and counting. Nucleic Acids Res 42:D259–D266. doi:10.1093/nar/gkt1047
Michelsen K, Yuan H, Schwappach B (2005) Hide and run. Arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins. EMBO Rep 6(8):717–722. doi:10.1038/sj.embor.7400480
Santoro B, Piskorowski RA, Pian P, Hu L, Liu H, Siegelbaum SA (2009) TRIP8b splice variants form a family of auxiliary subunits that regulate gating and trafficking of HCN channels in the brain. Neuron 62(6):802–813. doi:10.1016/j.neuron.2009.05.009
Nava C, Dalle C, Rastetter A, Striano P, de Kovel CG, Nabbout R, Cances C, Ville D, Brilstra EH, Gobbi G, Raffo E, Bouteiller D, Marie Y, Trouillard O, Robbiano A, Keren B, Agher D, Roze E, Lesage S, Nicolas A, Brice A, Baulac M, Vogt C, El Hajj N, Schneider E, Suls A, Weckhuysen S, Gormley P, Lehesjoki AE, De Jonghe P, Helbig I, Baulac S, Zara F, Koeleman BP, Euro ERESC, Haaf T, LeGuern E, Depienne C (2014) De novo mutations in HCN1 cause early infantile epileptic encephalopathy. Nat Genet 46(6):640–645. doi:10.1038/ng.2952
Tang B, Sander T, Craven KB, Hempelmann A, Escayg A (2008) Mutation analysis of the hyperpolarization-activated cyclic nucleotide-gated channels HCN1 and HCN2 in idiopathic generalized epilepsy. Neurobiol Dis 29(1):59–70. doi:10.1016/j.nbd.2007.08.006
Zerangue N, Schwappach B, Jan YN, Jan LY (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22(3):537–548
Wang HX, Gao WJ (2012) Prolonged exposure to NMDAR antagonist induces cell-type specific changes of glutamatergic receptors in rat prefrontal cortex. Neuropharmacology 62(4):1808–1822. doi:10.1016/j.neuropharm.2011.11.024
Gassmann M, Haller C, Stoll Y, Abdel Aziz S, Biermann B, Mosbacher J, Kaupmann K, Bettler B (2005) The RXR-type endoplasmic reticulum-retention/retrieval signal of GABAB1 requires distant spacing from the membrane to function. Mol Pharmacol 68(1):137–144. doi:10.1124/mol.104.010256
Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD (2001) An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci 21(9):3063–3072
Wenthold RJ, Sans N, Standley S, Prybylowski K, Petralia RS (2003) Early events in the trafficking of N-methyl-d-aspartate (NMDA) receptors. Biochem Soc Trans 31(Pt 4):885–888
Shikano S, Li M (2003) Membrane receptor trafficking: evidence of proximal and distal zones conferred by two independent endoplasmic reticulum localization signals. Proc Natl Acad Sci USA 100(10):5783–5788. doi:10.1073/pnas.1031748100
Much B, Wahl-Schott C, Zong X, Schneider A, Baumann L, Moosmang S, Ludwig A, Biel M (2003) Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem 278(44):43781–43786. doi:10.1074/jbc.M306958200
Brewster AL, Bernard JA, Gall CM, Baram TZ (2005) Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis 19(1–2):200–207. doi:10.1016/j.nbd.2004.12.015
Zha Q, Brewster AL, Richichi C, Bender RA, Baram TZ (2008) Activity-dependent heteromerization of the hyperpolarization-activated, cyclic-nucleotide gated (HCN) channels: role of N-linked glycosylation. J Neurochem 105(1):68–77. doi:10.1111/j.1471-4159.2007.05110.x
Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A (2001) Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem 268(6):1646–1652
Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA (2000) Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci 20(14):5264–5275
Hardel N, Harmel N, Zolles G, Fakler B, Klocker N (2008) Recycling endosomes supply cardiac pacemaker channels for regulated surface expression. Cardiovasc Res 79(1):52–60. doi:10.1093/cvr/cvn062
Yu HG, Lu Z, Pan Z, Cohen IS (2004) Tyrosine kinase inhibition differentially regulates heterologously expressed HCN channels. Pflugers Arch 447(4):392–400. doi:10.1007/s00424-003-1204-y
Zong X, Eckert C, Yuan H, Wahl-Schott C, Abicht H, Fang L, Li R, Mistrik P, Gerstner A, Much B, Baumann L, Michalakis S, Zeng R, Chen Z, Biel M (2005) A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotide-gated channels by Src kinase. J Biol Chem 280(40):34224–34232. doi:10.1074/jbc.M506544200
Iakoucheva LM, Radivojac P, Brown CJ, O’Connor TR, Sikes JG, Obradovic Z, Dunker AK (2004) The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res 32(3):1037–1049. doi:10.1093/nar/gkh253
Wong YH, Lee TY, Liang HK, Huang CM, Wang TY, Yang YH, Chu CH, Huang HD, Ko MT, Hwang JK (2007) KinasePhos 2.0: a web server for identifying protein kinase-specific phosphorylation sites based on sequences and coupling patterns. Nucleic Acids Res 35:W588–W594. doi:10.1093/nar/gkm322
Stepanchick A, McKenna J, McGovern O, Huang Y, Breitwieser GE (2010) Calcium sensing receptor mutations implicated in pancreatitis and idiopathic epilepsy syndrome disrupt an arginine-rich retention motif. Cell Physiol Biochem 26(3):363–374. doi:10.1159/000320560
Acknowledgments
This research was supported by a grant from the National Institutes of Health, EY020542, to S.A.B. and a Cornell College Dimensions Fellowship to D.M.Y. All authors contributed to the design, execution, and analysis of the experiments. Y.P. and S.A.B. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2014_1705_MOESM1_ESM.tif
Supplemental Fig. 1 Primers used to clone reporter: HCN1 constructs A) Cartoon of constructs from Fig. 1 with the location and names of cloning primers indicated. The portion of the primer in red corresponds to the 3′ end of the reporter, the portion in black corresponds to the HCN1 fragment. After splicing by overlap extension PCR the products were digested with AgeI and NotI then ligated into the XOP5.5 vector B) Sequences of primers (TIFF 324 kb)
18_2014_1705_MOESM2_ESM.tif
Supplemental Fig. 2 Localization of HCN1 relative to the ER in HEK293 cells Location of HA-HCN1WT (A) or HA-HCN1MUT (B) was determined by co-labeling with anti-HA antibodies (green) and the ER marker calnexin (red). Nuclei (blue), and scale bars, 10 μm (TIFF 422 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Pan, Y., Laird, J.G., Yamaguchi, D.M. et al. A di-arginine ER retention signal regulates trafficking of HCN1 channels from the early secretory pathway to the plasma membrane. Cell. Mol. Life Sci. 72, 833–843 (2015). https://doi.org/10.1007/s00018-014-1705-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1705-1