Abstract
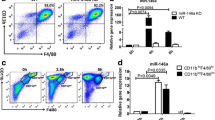
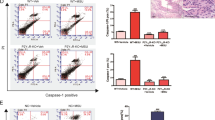
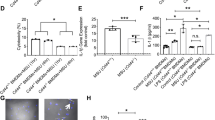
Cyclic GMP-dependent protein kinase II (cGKII; PRKG2) phosphorylates a variety of biological targets and has been identified as a gout-susceptible gene. However, the regulatory role of cGKII in triggering gout disease has yet to be clarified. Thus, we plan to explore the specific function of cGKII in macrophages related to gout disease. By using cGKII gene knockdown method, we detected macrophage M1/M2 polarization, phagocytosis, and their responses to stimulation by monosodium urate (MSU). cGKII was highly expressed in M1 phenotype, but not in M2, and cGKII knockdown significantly inhibited macrophage M1 polarization by decreasing M1 chemokine markers (CXCL10 and CCL2) and downregulating phagocytosis function. We further identified that cGKII-associated phagocytosis was mediated by upregulating toll-like receptor 2 (TLR2) expression, but not by TLR4. Mimicking gout condition by MSU treatments, we found that MSU alone induced cGKII and TLR2 expression with increased M1 polarization markers and phagocytosis activity. It means that cGKII knockdown significantly inhibited this MSU-induced cGKII-TLR2-phagocytosis axis. Our study showed that cGKII plays a key role in M1 polarization, especially in TLR2-mediated phagocytosis under MSU exposure. The findings provide evidence for the possible role of cGKII as an inflammation exciter in gout disease.
Key message
-
Gout-susceptible gene cGKII is necessary for macrophage M1 polarization.
-
cGKII regulates M1 phagocytosis function via TLR2.
-
Monosodium urate treatments increase cGKII expression and related function.
-
This study reveals the role of cGKII in enhancing gouty inflammatory responses.







Similar content being viewed by others
References
Chang SJ, Tsai MH, Ko YC, Tsai PC, Chen CJ, Lai HM (2009) The cyclic GMP-dependent protein kinase II gene associates with gout disease: identified by genome-wide analysis and case-control study. Ann Rheum Dis 68:1213–1219
Orstavik S, Solberg R, Tasken K, Nordahl M, Altherr MR, Hansson V, Jahnsen T, Sandberg M (1996) Molecular cloning, cDNA structure, and chromosomal localization of the human type II cGMP-dependent protein kinase. Biochem Biophys Res Commun 220:759–765
Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fassler R (1996) Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science 274:2082–2086
Campion EW, Glynn RJ, DeLabry LO (1987) Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med 82:421–426
Martin WJ, Shaw O, Liu X, Steiger S, Harper JL (2011) Monosodium urate monohydrate crystal-recruited noninflammatory monocytes differentiate into M1-like proinflammatory macrophages in a peritoneal murine model of gout. Arthritis Rheum 63:1322–1332
Martin WJ, Walton M, Harper J (2009) Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum 60:281–289
Chapman PT, Yarwood H, Harrison AA, Stocker CJ, Jamar F, Gundel RH, Peters AM, Haskard DO (1997) Endothelial activation in monosodium urate monohydrate crystal-induced inflammation: in vitro and in vivo studies on the roles of tumor necrosis factor alpha and interleukin-1. Arthritis Rheum 40:955–965
Landis RC, Yagnik DR, Florey O, Philippidis P, Emons V, Mason JC, Haskard DO (2002) Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis Rheum 46:3026–3033
Yagnik DR, Hillyer P, Marshall D, Smythe CD, Krausz T, Haskard DO, Landis RC (2000) Noninflammatory phagocytosis of monosodium urate monohydrate crystals by mouse macrophages. Implications for the control of joint inflammation in gout. Arthritis Rheum 43:1779–1789
Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, Akira S, Rock KL (2006) MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest 116:2262–2271
Scott P, Ma H, Viriyakosol S, Terkeltaub R, Liu-Bryan R (2006) Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J Immunol 177:6370–6378
Martinez FO, Sica A, Mantovani A, Locati M (2008) Macrophage activation and polarization. Front Biosci 13:453–461
Benoit M, Desnues B, Mege JL (2008) Macrophage polarization in bacterial infections. J Immunol 181:3733–3739
Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K (1980) Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer 26:171–176
Zhou X, Matavelli L, Frohlich ED (2006) Uric acid: its relationship to renal hemodynamics and the renal renin-angiotensin system. Curr Hypertens Rep 8:120–124
Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R (2005) Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum 52:2936–2946
Kingsbury SR, Conaghan PG, McDermott MF (2011) The role of the NLRP3 inflammasome in gout. J Inflamm Res 4:39–49
Mariathasan S, Monack DM (2007) Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 7:31–40
Swartling FJ, Ferletta M, Kastemar M, Weiss WA, Westermark B (2009) Cyclic GMP-dependent protein kinase II inhibits cell proliferation, Sox9 expression and Akt phosphorylation in human glioma cell lines. Oncogene 28:3121–3131
Dalbeth N, Pool B, Gamble GD, Smith T, Callon KE, McQueen FM, Cornish J (2010) Cellular characterization of the gouty tophus: a quantitative analysis. Arthritis Rheum 62:1549–1556
Czegley C, Biermann M, Weidner D, Hoffmann M, Herrmann M, Schauer C (2014) Monocytes and granulocytes orchestrate induction and resolution of inflammation in gout. Gout Hyperuricemia 1:88–93
Schorn C, Frey B, Lauber K, Janko C, Strysio M, Keppeler H, Gaipl US, Voll RE, Springer E, Munoz LE et al (2011) Sodium overload and water influx activate the NALP3 inflammasome. J Biol Chem 286:35–41
Amaral FA, Costa VV, Tavares LD, Sachs D, Coelho FM, Fagundes CT, Soriani FM, Silveira TN, Cunha LD, Zamboni DS et al (2012) NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum 64:474–484
Schorn C, Janko C, Latzko M, Chaurio R, Schett G, Herrmann M (2012) Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front Immunol 3:277
Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, Lell M, Manger B, Rech J, Naschberger E et al (2014) Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 20:511–517
Acknowledgments
This work was supported by the grants from the National Science Council (NSC103-2314-B-182A-072, NSC103-2314-B-390-001), and the Kaohsiung Medical University (KMU-M103004, KMU-TP103A24) in Taiwan, and the National Science Foundation in China (NSF 31371272).
Conflict of interest
None.
Author contributions
All authors were involved in writing the article or revising it critically for important intellectual content and approved the final version to be published. Dr. Liao WT, Chang SJ, and Chen CJ had full access to all of the data in the study and take responsibility for the accuracy of the data. Wei-Ting Liao contributed to the study design and experimental processing and wrote the Methods, Results, and Discussion. Huey-Ling You designed and performed the experiments. Changgui Li contributed to the technical support in cGKII pathway. Jan-Gowth Chang contributed to the organization of the study design and data analysis. Shun-Jen Chang wrote the Discussion, contributed to the study idea, and directed the study focus. Chung-Jen Chen contributed to the study design and result interpretation and wrote the Introduction and Discussion.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liao, WT., You, HL., Li, C. et al. Cyclic GMP-dependent protein kinase II is necessary for macrophage M1 polarization and phagocytosis via toll-like receptor 2. J Mol Med 93, 523–533 (2015). https://doi.org/10.1007/s00109-014-1236-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-014-1236-0