Abstract
Purpose
Loss of mitochondrial DNA (mtDNA) has been described in whole blood samples from a small number of patients with sepsis, but the underlying mechanism and clinical implications of this observation are not clear. We have investigated the cellular basis of the mtDNA depletion in sepsis, and determined clinical correlates with mtDNA depletion.
Methods
Whole blood samples were obtained from 147 consecutive patients with severe sepsis admitted to a General Critical Care Unit in a University Hospital and 83 healthy controls. In a separate study of 13 patients with severe sepsis, blood was obtained for immediate cell sorting by flow cytometry. MtDNA content was determined in whole blood DNA by PCR methods, and subsequently in the 13 samples where white cell subtypes were separated.
Results
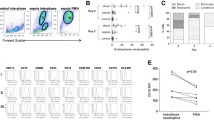
The mtDNA content of peripheral blood in human subjects was lower in patients with sepsis than controls (P < 0.0001). By studying leukocyte subsets in a subgroup of 13 patients, we showed that this was largely due to an increase in the proportion of circulating neutrophils, which contained ~3-fold less mtDNA than mononuclear leukocytes. However, isolated monocytes (P = 0.041) and lymphocytes (P = 0.021) from septic patients showed clear evidence of mtDNA depletion, which correlated with the APACHE II score (P = 0.015).
Conclusions
In severe sepsis much of the apparent whole blood mtDNA depletion is due to a change in the differential leukocyte count. However mtDNA depletion in mononuclear cells occurs in patients with sepsis and correlates with disease severity.





Similar content being viewed by others
References
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150
Singer M, Brealey D (1999) Mitochondrial dysfunction in sepsis. Biochem Soc Symp 66:149–166
Brealey D, Singer M (2003) Mitochondrial dysfunction in sepsis. Curr Infect Dis Rep 5:365–371
Fink MP (2001) Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin 17:219–237
Zeviani M, Di Donato S (2004) Mitochondrial disorders. Brain 127:2153–2172
Watts JA, Kline JA, Thornton LR, Grattan RM, Brar SS (2004) Metabolic dysfunction and depletion of mitochondria in hearts of septic rats. J Mol Cell Cardiol 36:141–150
Suliman HB, Carraway MS, Piantadosi CA (2003) Postlipopolysaccharide oxidative damage of mitochondrial DNA. Am J Respir Crit Care Med 167:570–579
Crouser ED, Julian MW, Huff JE, Struck J, Cook CH (2006) Carbamoyl phosphate synthase-1: a marker of mitochondrial damage and depletion in the liver during sepsis. Crit Care Med 34:2439–2446
Cote HC, Day AG, Heyland DK (2007) Longitudinal increases in mitochondrial DNA levels in blood cells are associated with survival in critically ill patients. Crit Care 11:R88
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 29:530–538
Pyle A, Taylor RW, Durham SE, Deschauer M, Schaefer AM, Samuels DC, Chinnery PF (2007) Depletion of mitochondrial DNA in leucocytes harbouring the 3243A->G mtDNA mutation. J Med Genet 44:69–74
Durham SE, Bonilla E, Samuels DC, DiMauro S, Chinnery PF (2005) Mitochondrial DNA copy number threshold in mtDNA depletion myopathy. Neurology 65:453–455
Veltri KL, Espiritu M, Singh G (1990) Distinct genomic copy number in mitochondria of different mammalian organs. J Cell Physiol 143:160–164
Hsieh YC, Frink M, Kawasaki T, Thobe BM, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH (2007) Downregulation of TLR4-dependent ATP production is critical for estrogen-mediated immunoprotection in Kupffer cells following trauma-hemorrhage. J Cell Physiol 211:364–370
Suliman HB, Welty-Wolf KE, Carraway MS, Schwartz DA, Hollingsworth JW, Piantadosi CA (2005) Toll-like receptor 4 mediates mitochondrial DNA damage and biogenic responses after heat-inactivated E. coli. Faseb J 19:1531–1533
Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU (2008) Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med 14:949–953
Medzhitov R, Janeway C Jr (2000) Innate immunity. N Engl J Med 343:338–344
Kumagai Y, Takeuchi O, Akira S (2008) Pathogen recognition by innate receptors. J Infect Chemother 14:86–92
Jeng JY, Yeh TS, Lee JW, Lin SH, Fong TH, Hsieh RH (2008) Maintenance of mitochondrial DNA copy number and expression are essential for preservation of mitochondrial function and cell growth. J Cell Biochem 103:347–357
D’Souza AD, Parikh N, Kaech SM, Shadel GS (2007) Convergence of multiple signaling pathways is required to coordinately up-regulate mtDNA and mitochondrial biogenesis during T cell activation. Mitochondrion 7:374–385
Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH (2008) Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 26:960–968
Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A (2005) Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol 78:325–337
Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365:63–78
Saada A, Shaag A, Mandel H, Nevo Y, Eriksson S, Elpeleg O (2001) Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat Genet 29:342–344
Bourdon A, Minai L, Serre V, Jais JP, Sarzi E, Aubert S, Chretien D, de Lonlay P, Paquis-Flucklinger V, Arakawa H, Nakamura Y, Munnich A, Rotig A (2007) Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat Genet 39:776–780
Cote HC, Brumme ZL, Craib KJ, Alexander CS, Wynhoven B, Ting L, Wong H, Harris M, Harrigan PR, O’Shaughnessy MV, Montaner JS (2002) Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med 346:811–820
Zsurka G, Baron M, Stewart JD, Kornblum C, Bos M, Sassen R, Taylor RW, Elger CE, Chinnery PF, Kunz WS (2008) Clonally expanded mitochondrial DNA mutations in epileptic individuals with mutated DNA polymerase gamma. J Neuropathol Exp Neurol 67:857–866
Acknowledgments
PFC is a Wellcome Trust Senior Fellow in Clinical Science who also receives funding from the EU FP6 program, EUmitocombat and MITOCIRCLE, the Parkinson's Disease Society (UK) and the Medical Research Council (UK). We thank Ian Dimmick and Rebecca Stewart for their assistance with the flow cytometry experiments. This work was principally funded by an unconditional research grant from the United States Army.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pyle, A., Burn, D.J., Gordon, C. et al. Fall in circulating mononuclear cell mitochondrial DNA content in human sepsis. Intensive Care Med 36, 956–962 (2010). https://doi.org/10.1007/s00134-010-1823-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1823-7