Abstract
Introduction and hypothesis
Human menopause transition and post-menopausal syndrome, driven by reduced ovarian activity and estrogen levels, are associated with an increased risk for symptoms including but not limited to sexual dysfunction, metabolic disease, and osteoporosis. Current treatments are limited in efficacy and may have adverse consequences, so investigation for additional treatment options is necessary. Previous studies have demonstrated that percutaneous tibial nerve stimulation (PTNS) and electro-acupuncture near the tibial nerve are minimally invasive treatments that increase vaginal blood perfusion or serum estrogen in the rat model. We hypothesized that PTNS would protect against harmful reproductive and systemic changes associated with menopause.
Methods
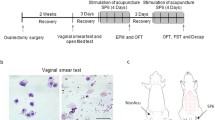
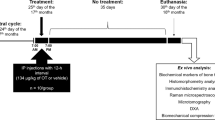
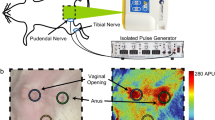
We examined the effects of twice-weekly PTNS (0.2 ms pulse width, 20 Hz, 2× motor threshold) under ketamine-xylazine anesthesia in ovariectomized (OVX) female Sprague-Dawley rats on menopause-associated physiological parameters including serum estradiol, body weight, blood glucose, bone health, and vaginal blood perfusion. Rats were split into three groups (n = 10 per group): (1) intact control (no stimulation), (2) OVX control (no stimulation), and (3) OVX stimulation (treatment group).
Results
PTNS did not affect serum estradiol levels, body weight, or blood glucose. PTNS transiently increased vaginal blood perfusion during stimulation for up to 5 weeks after OVX and increased areal bone mineral density and yield load of the right femur (side of stimulation) compared to the unstimulated OVX control.
Conclusions
PTNS may ameliorate some symptoms associated with menopause. Additional studies to elucidate the full potential of PTNS on menopause-associated symptoms under different experimental conditions are warranted.






Similar content being viewed by others
References
Whiteley J, DiBonaventura, daCosta M, Wagner J-S, Alvir J, Shah S. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J Women’s Health (2002). 2013;22(11):983–90. https://doi.org/10.1089/jwh.2012.3719.
Koebele SV, Bimonte-Nelson HA. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas. 2016;87:5–17. https://doi.org/10.1016/j.maturitas.2016.01.015.
Gracia CR, Freeman EW. Onset of the menopause transition: the earliest signs and symptoms. Obstet Gynecol Clin North Am. 2018;45(4):585–97. https://doi.org/10.1016/j.ogc.2018.07.002.
Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. 2005;3:47. https://doi.org/10.1186/1477-7525-3-47.
The 2017 hormone therapy position statement of The North American Menopause Society. Menopause (New York, NY). 2017;24(7):728–753. https://doi.org/10.1097/GME.0000000000000921
Gupta P, Ehlert MJ, Sirls LT, Peters KM. Percutaneous tibial nerve stimulation and sacral neuromodulation: an update. Current Urol Reports. 2015;16(2):4. https://doi.org/10.1007/s11934-014-0479-1.
de Groat WC, Tai C. Impact of bioelectronic medicine on the neural regulation of pelvic visceral function. Bioelectronic Med. 2015;2015:25–36. https://doi.org/10.15424/bioelectronmed.2015.00003.
Luan S, Williams I, Nikolic K, Constandinou TG. Neuromodulation: present and emerging methods. Front Neuroeng. 2014;7:27. https://doi.org/10.3389/fneng.2014.00027.
Levin RJ, Both S, Georgiadis J, Kukkonen T, Park K, Yang CC. The physiology of female sexual function and the pathophysiology of female sexual dysfunction (Committee 13A). J Sexual Med. 2016;13(5):733–59. https://doi.org/10.1016/j.jsxm.2016.02.172.
Zimmerman LL, Rice IC, Berger MB, Bruns TM. Tibial nerve stimulation to drive genital sexual arousal in an anesthetized female rat. J Sexual Med. 2018;15(3):296–303. https://doi.org/10.1016/j.jsxm.2018.01.007.
Zimmerman LL, Gupta P, O’Gara F, Langhals NB, Berger MB, Bruns TM. Transcutaneous electrical nerve stimulation to improve female sexual dysfunction symptoms: a pilot study. Neuromodul: J Int Neuromodul Soc. 2018;21(7):707–13. https://doi.org/10.1111/ner.12846.
Zimmerman LL, Mentzelopoulos G, Parrish HJ, Luma BD, Becker JB, Bruns TM. Modulating sexual behavior in female rats with tibial nerve electrical stimulation. Society for Neuroscience Annual Meeting [Abstract]. 2019. Retrieved from https://www.abstractsonline.com/pp8/#!/7883/presentation/70326
Li Y, Xu L, Qin Z. Effects of electroacupuncture stimulation of “Sanyinjiao” (SP 6) on hypothalamus’-pituitary-ovary axis in perimenopausal rats. Zhen ci yan jiu = Acupuncture Res. 2014;39(3):198–201.
Xia X, Hu L, Qin Z, Zhou J, Meng L, Li W-L, Zhang Y. Multicentral randomized controlled clinical trials about treatment of perimenopausal syndrome with electroacupuncture of sanyinjiao (SP 6). Zhen ci yan jiu = Acupuncture Res. 2008;33(4):262–6.
Zhao H, Tian Z, Cheng L, Chen B. Electroacupuncture enhances extragonadal aromatization in ovariectomized rats. Reprod Biol Endocrinol: RB&E. 2004;2:18. https://doi.org/10.1186/1477-7827-2-18.
Zhao H, Tian Z, Hao J, Chen B. Extragonadal aromatization increases with time after ovariectomy in rats. Reprod Biol Endocrinol. 2005;3(1):6. https://doi.org/10.1186/1477-7827-3-6.
Levin VA, Jiang X, Kagan R. Estrogen therapy for osteoporosis in the modern era. Osteoporos Int 2018;29(5):1049–1055. https://doi.org/10.1007/s00198-018-4414-z
Frye JB, Lukefahr AL, Wright LE, Marion SL, Hoyer PB, Funk JL. Modeling perimenopause in Sprague-Dawley rats by chemical manipulation of the transition to ovarian failure. Comp Med. 2012;62(3):193–202.
Moossdorff-Steinhauser HFA, Berghmans B. Effects of percutaneous tibial nerve stimulation on adult patients with overactive bladder syndrome: a systematic review. Neurourol Urodynam. 2013;32(3):206–14. https://doi.org/10.1002/nau.22296.
Kim SW, Jeong S-J, Munarriz R, Kim NN, Goldstein I, Traish AM. An in vivo rat model to investigate female vaginal arousal response. J Urol. 2004;171(3):1357–61. https://doi.org/10.1097/01.ju.0000109868.19569.d7.
Marson L, Cai R, Makhanova N. Identification of spinal neurons involved in the urethrogenital reflex in the female rat. J Comp Neurol. 2003;462(4):355–70. https://doi.org/10.1002/cne.10732.
Rice IC, Zimmerman LL, Ross SE, Berger MB, Bruns TM. Time-frequency analysis of increases in vaginal blood perfusion elicited by long-duration pudendal neuromodulation in anesthetized rats. Neuromodul: J Int Neuromodul Soc. 2017;20(8):807–15. https://doi.org/10.1111/ner.12707.
Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res Part B, Dev Reprod Toxicol. 2007;80(2):84–97. https://doi.org/10.1002/bdrb.20106.
OECD/OCDE. OECD Guideline for the testing of chemicals; uterotrophic bioassay in rodents: a short-term screening test for oestrogenic properties. 2007
Robbins A, Tom CATMB, Cosman MN, Moursi C, Shipp L, Spencer TM, et al. Low temperature decreases bone mass in mice: Implications for humans. Am J Phys Anthropol. 2018;167(3):557–68. https://doi.org/10.1002/ajpa.23684.
Goulet GC, Halonen NR, Koch LG, Britton SL, Zernicke RF, Kozloff KM. Osteoblast response to ovariectomy is enhanced in intrinsically high aerobic-capacity rats. Calcified Tissue Int. 2011;88(4):325–35. https://doi.org/10.1007/s00223-010-9457-x.
Li T, Ma Y, Zhang H, Yan P, Huo L, Hu Y, et al. Differential regulation of morphology and estrogen receptor-alpha expression in the vagina of ovariectomized adult virgin rats by estrogen replacement: a histological study. Int J Endocrinol. 2016;2016:1093512. https://doi.org/10.1155/2016/1093512.
Li T, Ma Y, Zhang H, Yan P, Huo L, Hu Y, et al. Estrogen replacement regulates vaginal innervations in ovariectomized adult virgin rats: a histological study. BioMed Res Int. 2017;2017:7456853. https://doi.org/10.1155/2017/7456853.
Vailati S, Melloni E, Riscassi E, Behr Roussel D, Sardina M. Evaluation of the effects of a new intravaginal gel, containing purified bovine colostrum, on vaginal blood flow and vaginal atrophy in ovariectomized rat. Sexual Med. 2013;1(2):35–43. https://doi.org/10.1002/sm2.8.
Levy M, Bassis CM, Kennedy E, Yoest KE, Becker JB, Bell J, et al. The rodent vaginal microbiome across the estrous cycle and the effect of genital nerve electrical stimulation. PloS one. 2020;15(3):e0230170. https://doi.org/10.1371/journal.pone.0230170.
Moazzam Z, Duke AR, Yoo PB. Inhibition and excitation of bladder function by tibial nerve stimulation using a wirelessly powered implant: an acute study in anesthetized cats. J Urol. 2016;196(3):926–33. https://doi.org/10.1016/j.juro.2016.04.077.
Thomas GP, Dudding TC, Bradshaw E, Nicholls RJ, Vaizey CJ. A pilot study to compare daily with twice weekly transcutaneous posterior tibial nerve stimulation for faecal incontinence. Colorectal Disease: Off J Assoc Coloproctol Great Britain Ireland. 2013;15(12):1504–9. https://doi.org/10.1111/codi.12428.
Alkis O, Sevim M, Güven Kartal İ, Baser A, İbrahim İvelik H, Aras B. Comparison of transcutaneous tibial nerve stimulation (TTNS) protocols for women with refractory overactive bladder (OAB): A prospective randomised trial. Int J Clin Practice. 2021;75(9):e14342. https://doi.org/10.1111/ijcp.14342.
Iqbal F, Collins B, Thomas GP, Askari A, Tan E, Nicholls RJ, Vaizey CJ. Bilateral transcutaneous tibial nerve stimulation for chronic constipation. Colorectal Disease: Off J Assoc Coloproctol Great Britain Ireland. 2016;18(2):173–8. https://doi.org/10.1111/codi.13105.
Gokce AH, Gokce FS. Effects of bilateral transcutaneous tibial nerve stimulation on constipation severity in geriatric patients: A prospective clinical study. Geriatrics Gerontol Intern. 2020;20(2):101–5. https://doi.org/10.1111/ggi.13822.
Martin-Garcia M. Effectiveness of bilateral percutaneous posterior tibial nerve stimulation for women with idiopathic overactive bladder. J Pelvic, Obstetric Gynaecol Physiother. 2017 53–57. Retrieved from https://thepogp.co.uk/_userfiles/pages/files/09_14301025_2.pdf
Nowicki M, Baum P, Kosacka J, Stockinger M, Klöting N, Blüher M, et al. Effects of isoflurane anesthesia on F-waves in the sciatic nerve of the adult rat. Muscle & Nerve. 2014;50(2):257–61. https://doi.org/10.1002/mus.24150.
Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci. 2001;69(10):1193–202. https://doi.org/10.1016/s0024-3205(01)01182-1.
Prando S, de Carneiro CG, Otsuki DA, Sapienza MT. Effects of ketamine/xylazine and isoflurane on rat brain glucose metabolism measured by (18) F-fluorodeoxyglucose-positron emission tomography. Eur J Neurosci. 2019;49(1):51–61. https://doi.org/10.1111/ejn.14252.
Lei H, Grinberg O, Nwaigwe CI, Hou HG, Williams H, Swartz HM, Dunn JF. The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res. 2001;913(2):174–9. https://doi.org/10.1016/s0006-8993(01)02786-x.
Flintoff K, Oh Rats! A Guide to Rat Anaesthesia for Veterinary Nurses and Technicians. N Z Vet Nurse 2014;22. Retrieved from https://www.nzvna.org.nz/site/nzvna/files/Quizzes/RatAnaesthesia.pdf
Origoni M, Leone Roberti Maggiore U, Salvatore S, Candiani M. Neurobiological mechanisms of pelvic pain. BioMed Res Int 2014;2014:903848. https://doi.org/10.1155/2014/903848
Paillard T. Regular muscle electrical stimulation could act favorably on bone mineral density in healthy aged subjects. Front Phys. 2018;9:1035. https://doi.org/10.3389/fphys.2018.01035.
Acknowledgements
We thank Teera Losch from the U-M Core Assay Facility lab for her assistance in estradiol kit selection and sample processing, Yesen Zhou and Christopher Fry in Jean Nemzek’s lab for their assistance with processing and storing samples, Ingrid Bergin from U-M In-Vivo Animal Core for her assistance with histology and expertise on the uterotrophic assay, Dana Jackson for his assistance with preliminary biomechanical testing, Jill Becker for her advice during experimental design, and the Unit for Laboratory Animal Medicine for their care of the animals. We would like to acknowledge support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases for the Michigan Integrative Musculoskeletal Health Core Center (P30 AR069620).
Author information
Authors and Affiliations
Contributions
J Xu: Project development, Data collection, Data analysis, Manuscript Writing. L Zimmerman: Project development. V Soriano: Data collection, Data analysis. G Mentzelopoulos: Data collection. E Kennedy: Data collection. E Bottorff: Data collection. C Stephen: Data collection, Data analysis. K Kozloff: Data collection, Data analysis. M Devlin: Data collection, Data analysis. T Bruns: Project Development, Data analysis, Manuscript writing
Corresponding authors
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 373 kb)
Rights and permissions
About this article
Cite this article
Xu, J.J., Zimmerman, L.L., Soriano, V.H. et al. Tibial nerve stimulation increases vaginal blood perfusion and bone mineral density and yield load in ovariectomized rat menopause model. Int Urogynecol J 33, 3543–3553 (2022). https://doi.org/10.1007/s00192-022-05125-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05125-5