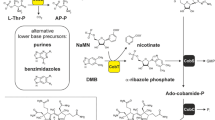
Abstract
The hydride carrier coenzyme F420 contains the unusual chromophore 7,8-didemethyl-8-hydroxy-5-deazariboflavin (FO). Microbes that generate F420 produce this FO moiety using a pyrimidine intermediate from riboflavin biosynthesis and the 4-hydroxyphenylpyruvate precursor of tyrosine. The fbiC gene, cloned from Mycobacterium smegmatis, encodes the bifunctional FO synthase. Expression of this protein in Escherichia coli caused the host cells to produce FO during growth, and activated cell-free extracts catalyze FO biosynthesis in vitro. FO synthase in the methanogenic euryarchaeon Methanocaldococcus jannaschii comprises two proteins encoded by cofG (MJ0446) and cofH (MJ1431). Both subunits were required for FO biosynthesis in vivo and in vitro. Cyanobacterial genomes encode homologs of both genes, which are used to produce the coenzyme for FO-dependent DNA photolyases. A molecular phylogeny of the paralogous cofG and cofH genes is consistent with the genes being vertically inherited within the euryarchaeal, cyanobacterial, and actinomycetal lineages. Ancestors of the cyanobacteria and actinomycetes must have acquired the two genes, which subsequently fused in actinomycetes. Both CofG and CofH have putative radical S-adenosylmethionine binding motifs, and pre-incubation with S-adenosylmethionine, Fe2+, sulfide, and dithionite stimulates FO production. Therefore a radical reaction mechanism is proposed for the biosynthesis of FO.




Similar content being viewed by others
Abbreviations
- AdoMet (SAM) :
-
S-adenosyl-l-methionine
- Compound 6 :
-
5-Amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione
- FO :
-
7,8-Didemethyl-8-hydroxy-5-deazariboflavin
- HPP :
-
4-Hydroxyphenylpyruvate
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Ashton WT, Brown RD (1980) Synthesis of 8-demethyl-8-hydroxy-5-deazariboflavins. J Heterocyclic Chem 17:1709–1712
Bacher A (1986) Heavy riboflavin synthase from Bacillus subtilis. Methods Enzymol 122:192–199
Bacher A, Eberhardt S, Fischer M, Kis K, Richter G (2000) Biosynthesis of vitamin B2 (riboflavin). Annu Rev Nutr 20:153–167
Blasi F, Fragomele F, Covelli I (1969) Thyroidal phenylpyruvate tautomerase. Isolation and characterization. J Biol Chem 244:4864–4870
Bult CJ, White O, Olsen GJ, Zhou L, Fleischmann RD, Sutton GG et al. (1996) Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science 273:1017–1140
Cahnmann HJ, Funakoshi K (1970) Model reactions for the biosynthesis of thyroxine. Nonenzymic formation of 3,5,3’-triiodothyronine from 4-hydroxy-3-iodophenylpyruvic acid, 3,5-diiodotyrosine, and oxygen. Biochemistry 9:90–98
Cheek J, Broderick JB (2001) Adenosylmethionine-dependent iron-sulfur enzymes: versatile clusters in a radical new role. J Biol Inorg Chem 6:209–226
Cheeseman P, Toms-Wood A, Wolfe RS (1972) Isolation and properties of a fluorescent compound, factor 420, from Methanobacterium strain M.o.H. J Bacteriol 112:527–531
Choi K-P, Bair TB, Bae Y-M, Daniels L (2001) Use of transposon Tn5367 mutagenesis and a nitroimidazopyran-based selection system to demonstrate a requirement for fbiA and fbiB in coenzyme F420 biosynthesis by Mycobacterium bovis BCG. J Bacteriol 183:7058–7066
Choi KP, Kendrick N, Daniels L (2002) Demonstration that fbiC is required by Mycobacterium bovis BCG for coenzyme F420 and FO biosynthesis. J Bacteriol 184:2420–2428
Cousins FB (1960) The prosthetic group of a chromoprotein from Mycobacteria. Biochim Biophys Acta 40:532–534
De Wit LEA, Eker APM (1987) 8-Hydroxy-5-deazaflavin-dependent electron transfer in the extreme halophile Halobacterium cutirubrum. FEMS Microbiol Lett 48:121–125
Duin EC, Lafferty ME, Crouse BR, Allen RM, Sanyal I, Flint DH, Johnson MK (1997) [2Fe-2S] to [4Fe-4S] cluster conversion in Escherichia coli biotin synthase. Biochemistry 36:11811–11820
Eberhardt S, Korn S, Lottspeich F, Bacher A (1997) Biosynthesis of riboflavin: an unusual riboflavin synthase of Methanobacterium thermoautotrophicum. J Bacteriol 179:2938–2943
Eirich LD, Vogels GD, Wolfe RS (1978) Proposed structure for coenzyme F420 from Methanobacterium. Biochemistry 17:4583–4593
Eisenreich W, Schwarzkopf B, Bacher A (1991) Biosynthesis of nucleotides, flavins, and deazaflavins in Methanobacterium thermoautotrophicum. J Biol Chem 266:9622–9631
Eker AP, Kooiman P, Hessels JK, Yasui A (1990) DNA photoreactivating enzyme from the cyanobacterium Anacystis nidulans. J Biol Chem 265:8009–8015
Felsenstein J (2001) PHYLIP (phylogeny inference package). In, 3.6a2.1 edn. Department of Genetics, University of Washington, Seattle
Finley KT (1974) The addition and substitution chemistry of quionines. In: Patai S (ed) The chemistry of the quinonoid compounds. Wiley, London, pp 877–1144
Frey PA (2001) Radical mechanisms of enzymatic catalysis. Annu Rev Biochem 70:121–148
Goyal RN, Kumar A, Jain N, Gupta P (1999) Electrochemical oxidation of 6-hydroxy-2,4,5-triaminopyrimidine at pyrolytic graphite electrode. Indian J Chem 38A:1015–1023
Graham DE, White RH (2002) Elucidation of methanogenic coenzyme biosyntheses: from spectroscopy to genomics. Nat Prod Rep 19:133–147
Graham DE, Xu H, White RH (2002) Identification of coenzyme M biosynthetic phosphosulfolactate synthase. A new family of sulfonate-biosynthesizing enzymes. J Biol Chem 277:13421–13429
Graupner M, White RH (2003) Methanococcus jannaschii coenzyme F420 analogs contain a terminal a-linked glutamate. J Bacteriol 185:4662–4665
Graupner M, Xu H, White RH (2002) The pyrimidine nucleotide reductase step in riboflavin and F420 biosynthesis in archaea proceeds by the eukaryotic route to riboflavin. J Bacteriol 184:1952–1957
Haase I, Mörtl S, Köhler P, Bacher A, Fischer M (2003) Biosynthesis of riboflavin in archaea. 6,7-dimethyl-8-ribityllumazine synthase of Methanococcus jannaschii. Eur J Biochem 270:1025–1032
Hemmerich P, Massey V, Fenner H (1977) Flavin and 5-deazaflavin: a chemical evaluation of ‘modified’ flavoproteins with respect to the mechanisms of redox biocatalysis. FEBS Lett 84:5–21
Hu XE, Yang HW, Wang XJ, Bai RS (2002) Electrohydrogenation of 4-amino-5-nitrosodimethyluracil with a foamed nickel cathode. J Appl Electrochem 32:321–328
Huang X, Holden HM, Raushel FM (2001) Channeling of substrates and intermediates in enzyme-catalyzed reactions. Annu Rev Biochem 70:149–180
Isabelle D, Simpson DR, Daniels L (2002) Large-scale production of coenzyme F420-5,6 by using Mycobacterium smegmatis. Appl Environ Microbiol 68:5750–5755
Kaneko T, Nakamura Y, Wolk CP, Kuritz T, Sasamoto S, Watanabe A et al. (2001) Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res 8:205–213; 227–253
Kern R, Keller PJ, Schmidt G, Bacher A (1983) Isolation and structural identification of a chromophoric coenzyme F420 fragment from culture fluid of Methanobacterium thermoautotrophicum. Arch Microbiol 136:191–193
Kulzer R, Pils T, Kappl R, H¸ttermann J, Knappe J (1998) Reconstitution and characterization of the polynuclear iron-sulfur cluster in pyruvate formate-lyase-activating enzyme. Molecular properties of the holoenzyme form. J Biol Chem 273:4897–4903
Larsen MH (2000) Some common methods in mycobacterial genetics. In: Hatfull GF, Jacobs WRJ (eds) Molecular genetics of mycobacteria. ASM Press, Washington, DC, p 316
Li H, Graupner M, Xu H, White RH (2003) CofE catalyzes the addition of two glutamates to F420-0 in F420 coenzyme biosynthesis in Methanococcus jannaschii. Biochemistry 42:9771–9778
Lin X-L, White RH (1986) Occurrence of coenzyme F420 and its γ-monoglutamyl derivative in nonmethanogenic archaebacteria. J Bacteriol 168:444–448
McCready S, Marcello L (2003) Repair of UV damage in Halobacterium salinarum. Biochem Soc Trans 31:694–698
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Plainview, New York
Plaut GWE, Harvey RA (1971) The enzymatic synthesis of riboflavin. Methods Enzymol 18B:515–538
Reuke B, Korn S, Eisenreich W, Bacher A (1992) Biosynthetic precursors of deazaflavins. J Bacteriol 174:4042–4049
Seo HS, Koo YJ, Lim JY, Song JT, Kim CH, Kim JK, Lee JS, Choi YD (2000) Characterization of a bifunctional enzyme fusion of trehalose-6-phosphate synthetase and trehalose-6-phosphate phosphatase of Escherichia coli. Appl Environ Microbiol 66:2484–2490
Slesarev AI, Mezhevaya KV, Makarova KS, Polushin NN, Shcherbinina OV, Shakhova VV et al. (2002) The complete genome of hyperthermophile Methanopyrus kandleri AV19 and monophyly of archaeal methanogens. Proc Natl Acad Sci USA 99:4644–4649
Sofia HJ, Chen G, Hetzler BG, Reyes-Spindola JF, Miller NE (2001) Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods. Nucleic Acids Res 29:1097–1106
Tabor S, Richardson CC (1985) A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA 82:1074–1078
Taylor EC, Jr., Loux HM, Falco E, Hitchings GH (1955) Pyrimidopteridines by oxidative self-condensation of aminopyrimidines. J Am Chem Soc 77:2243–2248
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Van QL, Schwarzkopf B, Bacher A, Keller PJ, Lee S, Floss HG (1985) Biosynthesis of 7,8-didemethyl-8-hydroxy-5-deazariboflavin, the chromophoric moiety of coenzyme F420. J Am Chem Soc 107:8300–8301
Walsh C (1986) Naturally occurring 5-deazaflavin coenzymes: biological redox roles. Acc Chem Res 19:216–221
Acknowledgements
This work was supported by a U.S. National Science Foundation grant awarded to D.E.G. in 2002 and NSF Grant MCB 0231319 to R.H.W.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graham, D.E., Xu, H. & White, R.H. Identification of the 7,8-didemethyl-8-hydroxy-5-deazariboflavin synthase required for coenzyme F420 biosynthesis. Arch Microbiol 180, 455–464 (2003). https://doi.org/10.1007/s00203-003-0614-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-003-0614-8