Abstract
Rationale
Drug-induced parkinsonism (DIP) is one of the main causes of treatment drop-out in schizophrenic patients causing a high incidence of relapse that leads patients to a bad clinical prognosis. The dopaminergic nigrostriatal pathway is involved in the movement control, so the study of the dopamine transporter (DAT) could be of great value to determine its implication in the appearance of DIP.
Objective
The goal of the study is to determine the striatal DAT binding assessed with [123I] FP-CIT SPECT in first-episode neuroleptic-naive schizophrenic in-patients with DIP after short-term antipsychotic treatment.
Method
The [123I] FP-CIT binding ratios of ten schizophrenic in-patients who developed DIP during the first 4-week period of risperidone treatment (6±2 mg/day) were compared with ten schizophrenic in-patients treated with the same doses of risperidone and who do not developed DIP and with ten age-matched healthy subjects. Quantitative analyses of SPECTs were performed using regions of interest located in caudate, putamen and occipital cortex. Parkinsonism was assessed by the Simpson–Angus Scale and the psychopathological status by the Clinical General Impression and Positive and Negative Syndrome Scales.
Results
Whole striatal [123I] FP-CIT binding ratios were significantly lower in patients with and without DIP than in healthy subjects (p<0.001). This was also observed in whole putamen (p<0.001) and caudate nucleus (p<0.001). Females showed higher whole striatal [123I] FP-CIT binding ratios than males (p<0.05). No differences in psychopathological scales were observed between patients with and without DIP.
Conclusion
Our first-episode schizophrenic patients with and without DIP after short-term risperidone treatment have a decreased striatal DAT binding assessed with [123I] FP-CIT. This alteration could be related to the schizophrenic disease or may be secondary to the antipsychotic treatment.
Similar content being viewed by others
Introduction
Drug-induced parkinsonism (DIP) is frequently observed (30–54% of patients) with typical antipsychotics. The percentage of patients with DIP drops to 15–20% in subjects who are treated with atypical antipsychotics. However, in both cases, DIP is mainly related to striatal D2 receptor blockade (Jibson and Tandon 1998). In general, this side effect is dose-dependent, and in most cases, patients can recover when antipsychotic is withdrawn (Miller and Jankovic 1990). However, a high prevalence of parkinsonian symptoms (PS) in schizophrenic naive patients was classically described by Bleuler (1950) and Kraeplin (1919) even before the introduction of antipsychotic drugs into clinical management. A few authors such as Caliguri et al. (1993) and McCreadie et al. (1996, 2002) have determined that the prevalence of PS in schizophrenic naive patients is about 14–24% and the prevalence of spontaneous dyskinesia is about 35–57%, depending on sex and age, being higher in female and in those older than 60 years. These data contrast with the prevalence of PS in the general population which is about 1% (Wolff and O’Driscoll 1999) and suggest that the association between schizophrenia and PS is not incidental.
On the other hand, it has been described in some patients with pharmacological parkinsonism induced by neuroleptics that clinical manifestations did not remit completely when the neuroleptic treatment was discontinued, suggesting an unmasked presynaptic nigrostriatal dysfunction due to a preclinical PD (Rajput et al. 1982; Burn and Brooks 1993).
The introduction of a new widely available SPECT radioligand ([123I] FP-CIT) that binds to the dopamine transporters (DAT), a presynaptic membrane protein located in dopaminergic nigrostriatal neurons, has proved useful to differentiate Parkinson’s disease (PD) from normal subjects, including those patients with early or asymptomatic PD (Tissingh et al. 1998).
Our hypothesis is that schizophrenic patients treated with antipsychotic that develop DIP may have a presynaptic dopamine dysfunction.
Thus, the aim of the study was to determine the striatal DAT binding, assessed with [123I] FP-CIT SPECT, in first-episode schizophrenic patients who developed DIP within the first 4 weeks of antipsychotic treatment.
Materials and methods
Subjects
To be eligible for screening, first-episode neuroleptic-naive schizophrenic in-patients underwent a Structured Diagnostic Interview (SCID) (First et al. 1994) for DSM-IV (American Psychiatric Association 1994) and had to meet the criteria for schizophrenia or schizophreniform disorder. The first ten consecutive patients (six men, four women; aged 26±4.5) which developed PS (DIP group) assessed by Simpson–Angus scale (S-A) (Simpson and Angus 1970) during the first 4-week period of risperidone treatment and the first ten consecutive patients (eight men, two women; aged 26±5) which did not develop PS (NoDIP group) after the same time period of treatment were included in the study. Excluded were patients taking medications with potential central nervous system effects, those with neurological disorders and those with any history of mania, hypomania, bipolar disorders, current substance dependence or when drug tests were positive. Drug testing was done at the recruitment. Included only were patients and healthy controls with negative testing, except for cannabis.
A group of ten age-matched healthy subjects (six men, four women; aged 27±4.3) was also evaluated using the SCID interview and had no DSM-IV axis I diagnosis. Demographic characteristics of all subjects are listed in Table 1. The study was approved by the ethical committee, and all patients (or their legal representative) and healthy subjects gave their written informed consent to participate in the study.
Study design and clinical assessment
Patients were consecutively included and were treated with 6±2 mg/day of risperidone, following the clinical guidelines of our Psychiatric Institute. No anticholinergic or other concomitant treatment was allowed. So, those who developed an acute dystonia and required anticholinergic treatment were excluded.
Patients that scored 3 or greater in the S-A scale during follow-up were included in the DIP group. The others were included in the NoDIP group.
Psychopathological status was assessed by Clinical General Impression Scale (CGI) (Guy 1976) and Positive and Negative Syndrome Scale (PANSS) (Kay et al. 1987). All the scales were assessed before treatment (baseline) and once a week during the 4-week follow-up. [123I] FP-CIT SPECTs of the selected patients were performed after 4 weeks of treatment. Healthy subjects’ SPECTs were performed when recruited.
SPECT procedure
One hour before 185-MBq [123I] FP-CIT (DaTSCAN, GE Healthcare) injection, all subjects received 300 mg of Lugol solution to block thyroid gland of free radioactive iodine.
SPECTs were performed 3 h after radiotracer injection using a rotating dual-head gammacamera (Helix, G.E.M.S.), fitted with a high-resolution fan beam collimator. A hundred and twenty 22-s frames were collected in a 360° circular orbit, step and shoot mode, using a 128×128 matrix.
Image data were processed on an SP1 Elscint computer (Apex SP-X, software version 3.12). Reconstruction was performed using filtered back projection with a Metz filter (FWHM=10 mm; power factor=3) that allowed partial recovering of the spatial resolution. The pixel size was 3.9 mm, and no attenuation correction was performed. All images from SPECT studies were reconstructed with a width of 1 pixel.
Quantitative analysis was performed by the method described by Walker et al. (2002) using circular regions of interest (ROIs) in three consecutive 3.9-mm-thick oblique slices parallel to the fronto-cerebellum plane (Fig. 1). Regions were placed on the caudate nucleus (4 pixels ∅; total volume 2.2 ml), anterior putamen, medial putamen, posterior putamen (2 pixels ∅; total volume 0.6 ml each one) and occipital cortex (non-specific binding; 6 pixels ∅; total volume 5.0 ml). ROIs were always placed by the same nuclear medicine physician in the anatomical regions previously described. They were fitted in the middle of the higher activity zone of each of the regions and far away from the edge, just to minimise the partial volume effects. For each hemisphere, the [123I] FP-CIT binding ratios (r) were obtained as:
where m S and m O are the mean counts per pixel in the striatal and occipital ROIs, respectively. This formula was used to obtain whole striatum (S=W), caudate nucleus (S=C), whole putamen (S=P), anterior putamen (S=AP), medial putamen (S=MP) and posterior putamen (S=PP) ratios by calculating the average counts of all the corresponding regions. Whole striatum was the sum of the caudate nucleus ROI plus all ROIs of entire putamen nucleus.
Statistical analysis
Differences between DIP, NoDIP and healthy groups, for each ROI (caudate, putamen, whole striatum), left and right hemispheres and psychopathological and S-A scales, were assessed using non-parametric tests (Kruskal–Wallis and Mann–Whitney U-test). The same comparison was made between male and female. The statistical significance level was defined as p<0.05 and was adjusted by the Bonferroni correction when a large number of comparisons were made. Correlations between variables were measured using two-tailed Spearman’s rho (ρ). All statistical analyses were carried out with SPSS 11.0 for Windows.
Results
No statistical differences were found in mean baseline and endpoint psychopathological scores (CGI, PANSS-Negative, PANSS-Positive, PANSS-General) between DIP and NoDIP groups (Table 2).
[123I] FP-CIT SPECT imaging
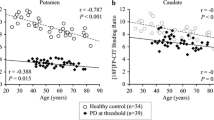
Whole striatum [123I] FP-CIT binding ratios were significantly lower in DIP and NoDIP groups than in healthy group (p<0.001) (Fig. 2). This was also observed in whole putamen (p<0.001), caudate nucleus (p<0.001), anterior putamen (p<0.05) and posterior putamen (p<0.001) (Table 3).
Females showed higher whole striatal [123I] FP-CIT binding ratios than males (p<0.05) (Fig. 3), but this difference was only observed in the healthy group (p<0.032) and in the NoDIP group (p<0.037). In the DIP group, although females obtained higher [123I] FP-CIT binding ratios than males, the difference did not reach statistical significance (p<0.136) (Table 4).
Simpson–Angus Scale and [123I] FP-CIT SPECT imaging
No statistical difference was found in mean baseline S-A scale score between DIP and NoDIP patients. A difference was found in mean endpoint S-A scale score between both groups of patients (p<0.001) (Table 2).
In the DIP group, four patients presented a mild but detectable parkinsonian sign (S-A score>0, <3) before treatment started. This sign was predominantly bradykinesia, which worsened during the 4-week treatment period. The remaining showed increases in the S-A scale over three after 1 week of antipsychotic treatment and worsened progressively, having the higher scores during the last week of treatment.
Two patients in the NoDIP group presented mild trends of bradykinesia before treatment started (S-A score>0, <3). However, after 4-week treatment, the S-A scale scored less than three points. Two more patients showed some type of parkinsonism during the follow-up period, but none of them reached the three-point level in the S-A scale to qualify for inclusion in the DIP group. Patients of this group were followed-up for four additional weeks, and none of them developed parkinsonism.
S-A scale score did not correlate with [123I] FP-CIT binding ratios neither in the whole striatum nor in the caudate nucleus or putamen in both groups of patients.
Discussion
Extrapyramidal symptoms are one of the main causes of treatment drop-out that cause a high incidence of relapse with frequent admissions into psychiatric hospitals. Consequently, it leads patients to a bad clinical prognosis (Corrigan et al. 1990). It is well known that the cause of DIP in most treated schizophrenic patients could be explained by the excessive postsynaptic D2 blockade, resulting in a relative increase in cholinergic activity. The study of the nigrostriatal DAT binding could be of great value to determine if PS developed by schizophrenic patients may also be due to a presynaptic dopamine pathway dysfunction (Burn and Brooks 1993). This could explain the higher prevalence of PS observed in schizophrenic patients in the pre-antipsychotic era (Bleuler 1950; Kraeplin 1919).
The are many references which demonstrate a consistent decrease in striatal DAT binding in patients with symptomatic PD and other degenerative parkinsonian syndromes (Booij et al. 1999; Catafau and Tolosa 2004). Integrity in the striatal DAT binding has been described in pure forms of pharmacological parkinsonism (Verhoeff 1999; Booij et al. 1999). However, patients with pharmacological parkinsonism associated with presynaptic nigrostriatal dysfunction determined by PET or SPECT have poor outcomes after drug withdrawal, probably because they suffer from a preclinical PD (Burn and Brooks 1993).
In this study, we found a significant lower DAT binding in schizophrenic patients with DIP when compared with an age-matched healthy subjects group, but we did not find differences between patients with and without DIP. The lower striatal DAT binding would suggest a presynaptic dysfunction that could explain the higher prevalence of PS observed in the general schizophrenic population (McCreadie et al. 1996, 2002; Peralta et al. 2002). This finding, which is in agreement with a study of chronic schizophrenic patients that found lower striatal [18F] CFT binding in schizophrenic patient compared with healthy subjects (Laakso et al. 2001), might be attributed to a higher loss of dopamine neurone terminals or a decrease in the DAT expression (Lieberman et al. 1990). In line with our findings, another study using [123I] IPT found a lower striatal DAT binding in patients with schizophrenia than in a healthy subjects group (Tatsch et al. 1999)
In contrast with our observation, other studies using [18F] CFT, [123I] β-CIT and [123I] FP-CIT did not find differences between groups of patients and healthy controls (Laakso et al. 2000; Laruelle et al. 2000; Lavalaye et al. 2001). Even a recent SPECT study (Sjoholm et al. 2004) with [123I] β-CIT has shown presynaptic DAT binding increase in chronic schizophrenic patients, probably due to biological adaptation to chronic D2 receptor blockade induced by neuroleptics. Thus, it is conceivable to explain this discrepancy due to the heterogeneity in the age range, illness duration of the samples, kind and length of treatments, the PET or SPECT radiotracers used and the different methods of quantification applied.
In order to avoid possible interactions between DAT and the various treatments used, in our study, all patients were treated with similar doses of risperidone and no other treatments, such as anticholinergics, were allowed. We considered this aspect because some authors have described a reduced presynaptic dopamine reuptake after anticholinergic treatment (Vander et al. 1995). On the contrary, null effects of antipsychotics in human or animal DAT have been published (Rivest et al. 1995; Reader et al. 1998; Richelson and Pfenning 1984; Gordon et al. 1996; Valchar and Hanbauer 1993), although the role of this medication in striatal DAT binding cannot be totally excluded.
Females, except for DIP group, showed statistically significantly higher uptake ratios when compared with age-matched males. However, for females from DIP group, there was also a trend to have higher binding ratios than males from DIP group. The lack of statistical differences may be a type II error. Our findings are in agreement with previous studies on animal neuroreceptors (Rivest et al. 1995) and human [18F] DOPA (Ernst et al. 1998) and [123I] FP-CIT studies (Lavalaye et al. 2000, 2001). The brain estrogen impregnation may determine an increase in neuronal receptors and/or brain metabolism in females entailing an important gender effect on striatal DAT binding (Seeman 1997).
Regarding the S-A scale scores, we did not find any correlation with the [123I] FP-CIT binding ratios in DIP and NoDIP groups. Moreover, no difference was found either between patients who scored in the baseline or those who did not score. The lack of correlation could be explained by the previously mentioned presynaptic striatal dysfunction in both groups of patients.
The psychopathological scales scores (CGI, PANSS-P, PANSS-N, PANSS-G) did not show differences between groups. All patients were first-episode schizophrenic patients, with similar time of illness evolution and treated with similar doses of risperidone during the follow-up period. The only difference between them was the presence of PS, and therefore, lack of differences between them was expected.
In conclusion, first-episode schizophrenic patients with and without DIP after short-term risperidone treatment have a decreased striatal DAT binding assessed with [123I] FP-CIT. This alteration could be related to the schizophrenic disease or may be secondary to the antipsychotic treatment. Further studies performing a two-step design with pre- and post-treatment striatal DAT binding measurements should be done.
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders (4th ed). Washington, USA
Bleuler E (1950) Dementia praecox or the group of schizophrenias. International University Press, New York
Booij J, Tissingh G, Winogrodzca A, Van Royen EA (1999) Imaging of the dopaminergic neurotransmission system using single-photon emission tomography and positron emission tomography in patients with parkinsonism. Eur J Nucl Med 26:171–182
Burn DJ, Brooks DJ (1993) Nigral dysfunction in drug-induced parkinsonism: an 18F-DOPA PET study. Neurology 43:552–556
Caliguri M, Lohr J, Jeste D (1993) Parkinsonism in neuroleptic-naive schizophrenic patients. Am J Psychiatry 150:1343–1348
Catafau A, Tolosa E (2004) Impact of dopamine transporter SPECT using 123I-ioflupane on diagnosis and management of patients with clinically uncertain parkinsonian syndromes. Mov Disord 19:1175–1182
Corrigan PW, Liberman RP, Engel JD (1990) From noncompliance to collaboration in the treatment of schizophrenia. Hosp Community Psychiatry 41:1203–1211
Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM (1998) DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomography study. J Neurosci 18:5901–5907
First MB, Spitzer RL, Williams JBW, Gibbon M (1994) Structured clinical interview for DSM-IV—patients edition (SCID-P). American Psychiatric Press, Washington, DC
Gordon I, Weizman R, Rehavi M (1996) Modulatory effect of agents active in the presynaptic dopaminergic system on the striatal dopamine transporter. Eur J Pharmacol 298:27–30
Guy W (1976) Early clinical drug evaluation unit (ECDEU), National Institute of Mental Health 76:338. Rockville, USA
Jibson MD, Tandon R (1998) New atypical antipsychotic medications. J Psychiatry Res 32:215–228
Kay SR, Fisbein S, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:262–273
Kraeplin E (1919) Dementia praecox and paraphrenia. University of Edinburgh, Edinburgh
Laakso A, Vilkman H, Alakare B, Haaparanta M, Bergman J, Solin O, Peurasaari J, Räkköläinen V, Syvälahti E, Hietala J (2000) Striatal dopamine transporter binding in neuroleptic naïve patients with schizophrenia studied with PET. Am J Psychiatry 157:269–271
Laakso A, Bergman J, Haaparanta M, Vilkman H, Solin O, Sivälahti E, Hietala J (2001) Decreased striatal dopamine transporter binding in vivo in chronic schizophrenia. Schizophr Res 52:115–120
Laruelle M, Abi-Dargham A, Van Dyck C, Gil R, D’Souza D, Krystal J, Seibyl J, Baldwin R, Innis R (2000) Dopamine and serotonin transporter in patients with schizophrenia: an imaging study with 123I-βCIT. Biol Psychiatry 47:371–379
Lavalaye J, Booij J, Reneman L, Habraken J, Van Royen E (2000) Effect of age and gender on dopamine transporter imaging with [123I] FP-CIT SPECT in healthy volunteers. Eur J Nucl Med 27:867–869
Lavalaye J, Linszen DH, Booij J, Dingemans PM, Reneman L, Habraken J, Gersons B, Van Royen E (2001) Dopamine transporter density in young patients with schizophrenia assessed with [123I] FP-CIT SPECT. Schizophr Res 47:59–67
Lieberman JA, Kinon BL, Loebel AD (1990) Dopaminergic mechanisms in idiopathic and drug-induced psychoses. Schizophr Bull 16:97–110
McCreadie RG, Thara R, Kamath D, Padmavati R, Latha S, Mathrubootham N, Menon S (1996) Abnormal movements in never medicated Indian patients with schizophrenia. Br J Psychiatry 168:221–226
McCreadie R, Padmavati R, Thara R, Srinivasan TN (2002) Spontaneous dyskinesia and parkinsonism in never-medicated, chronically ill patients with schizophrenia: 18-month follow-up. Br J Psychiatry 181:135–137
Miller LG, Jankovic J (1990) Neuroleptic approach to drug-induced movement disorders: a study of 125 patients. South Med J 83:525–532
Peralta V, Cuesta M, Campos MS (2002) Sintomas extrapiramidales en pacientes esquizofrénicos nunca tratados con neurolépticos. Aula Méd Psiquiátr 4:269–280
Rajput AH, Rozdilsky B, Hornykiewicz O, Shannak K, Lee T, Seeman P (1982) Reversible drug-induced parkinsonism. Clinicopathologic study of two cases. Arch Neurol 10:644–646
Reader TA, Ase AR, Huang N, Hebert C, Van Gelder NM (1998) Neuroleptics and dopamine transporters. Neurochem Res 23:73–80
Richelson E, Pfenning M (1984) Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: most antidepressants selectively block norepinephrine uptake. Eur J Pharmacol 104:277–286
Rivest R, Falardeau P, Di Paolo T (1995) Brain dopamine transporter: gender differences and effect of chronic haloperidol. Brain Res 692:269–272
Seeman MV (1997) Psychopathology in women and men: focus on female hormones. Am J Psychiatry 154:1641–1647
Simpson GM, Angus JW (1970) A rating scale for extra-pyramidal side effects. Acta Psychiatr Scand, Suppl 212:11–19
Sjoholm H, Bratlid T, Sundsfjord J (2004) 123I β-CIT SPECT demonstrates increased presynaptic dopamine transporter binding sites in basal ganglia in vivo in schizophrenia. Psychopharmacology 173:27–31
Tatsch K, Scherer J, Linke R, Kerner M, Hahn K (1999) Decrease of dopamine transporter binding in neuroleptic-free schizophrenic patients assessed with IPT-SPECT (abstr.) J Nucl Med 40:31
Tissingh G, Booij J, Bergmans P, Winogrodzka A, Janssen AG, Van Royen EA, Stoof JC, Wolters E (1998) Iodine-123-N-omega-fluoropropyl-2beta-carbomethoxy-ebeta-(4-iodophenyl)-tropane SPECT in healthy controls and early-stage, drug-naïve Parkinson’s disease. J Nucl Med 39:1143–1148
Valchar M, Hanbauer I (1993) Comparison of 3HWIN 35,428 binding, a marker for dopamine transporter, in embryonic mesencephalic neuronal cultures with striatal membranes of adult rats. J Neurochem 60:469–476
Vander T, Kilbourn M, Desmond T, Kuhl D, Frey K (1995) The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. Eur J Pharmacol 294:577–583
Verhoeff NPLG (1999) Radiotracers imaging of dopaminergic transmission in neuropsychiatric disorders. Psychopharmacology 147:217–249
Walker Z, Costa DC, Walker RWH, Shaw K, Gacinovic S, Stevens T, Livingston G, Ince P, McKeith IG, Katona CLE (2002) Differentiation of dementia with Lewy bodies from Alzheimer’s disease using a dopaminergic presynaptic ligand. J Neurol Neurosurg Psychiatry 73:134–140
Wolff A, O’Driscoll G (1999) Motor deficits and schizophrenia: the evidence from neuroleptic-naïve patients and populations at risk. J Psychiatry Neurosci 24:304–314
Acknowledgements
Grants from Hospital Clinic of Barcelona (Premi Fi de Residencia 2001), Marató TV3 (Enfermedades Psiquiátricas Graves; 2001), Fondo de Investigación Sanitaria of the Spanish Ministerio de Sanidad y Consumo (contract no. PI020485) and Amersham Health are gratefully acknowledged. Thanks to Mr. J Lara, Ph.D., for his English editorial assistance and Mr. L Jover, Ph.D., for his statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mateos, J.J., Lomeña, F., Parellada, E. et al. Decreased striatal dopamine transporter binding assessed with [123I] FP-CIT in first-episode schizophrenic patients with and without short-term antipsychotic-induced parkinsonism. Psychopharmacology 181, 401–406 (2005). https://doi.org/10.1007/s00213-005-2250-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-2250-2