Abstract
Rationale
Addiction is characterized by persistence to seek drug reinforcement despite negative consequences. Drug-induced aberrations in approach and avoidance processing likely facilitate the sustenance of addiction pathology. Currently, the effects of repeated drug exposure on the resolution of conflicting approach and avoidance motivational signals have yet to be thoroughly investigated.
Objective
The present study sought to investigate the effects of cocaine pre-exposure on conflict resolution using novel approach-avoidance paradigms.
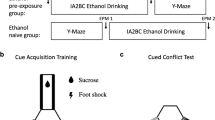
Methods
We used a novel mixed-valence conditioning paradigm to condition cocaine-pre-exposed rats to associate visuo-tactile cues with either the delivery of sucrose reward or shock punishment in the arms in which the cues were presented. Following training, exploration of an arm containing a superimposition of the cues was assessed as a measure of conflict resolution behavior. We also used a mixed-valence runway paradigm wherein cocaine-pre-exposed rats traversed an alleyway toward a goal compartment to receive a pairing of sucrose reward and shock punishment. Latency to enter the goal compartment across trials was taken as a measure of motivational conflict.
Results
Our results reveal that cocaine pre-exposure attenuated learning for the aversive cue association in our conditioning paradigm and enhanced preference for mixed-valence stimuli in both paradigms.
Conclusions
Repeated cocaine pre-exposure allows appetitive approach motivations to gain greater influence over behavioral output in the context of motivational conflict, due to aberrant positive and negative incentive motivational processing.






Similar content being viewed by others
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Publishing, Arlington
Bellone C, Luscher C (2006) Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci 9(5):636–641
Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 191(3):391–431
Bordreau AC, Wolf ME (2005) Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci 25(40):9144–9151
Borgland SL, Malenka RC, Bonci A (2004) Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci 24(34):7482–7490
Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Bonci A (2008) Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron 59(2):288–297
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Wolf ME (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454(7200):118–121
Deroche V, Le Moal M, Piazza PV (1999) Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci 11(8):2731–2736
Ettenberg A (2004) Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev 27(8):721–728
Ettenberg A, Geist TD (1991) Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology (Berl) 103(4):455–461
Everitt B, Robbins T (2013) From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev 37(9 Pt A):1946–1954
Fiorino DF, Philips AG (1999) Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after d-amphetamine-induced behavioral sensitization. J Neurosci 19(1):456–463
Gratton A, Wise RA (1994) Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous cocaine self-administration in rats. J Neurosci 14(7):4130–4146
Harmer CJ, Phillips GD (1998) Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behav Pharmacol 9(4):299–308
Harmer CJ, Phillips GD (1999) Enhanced dopamine efflux in the amygdala by a predictive, but not a non-predictive, stimulus: facilitation by prior repeated D-amphetamine. Neuroscience 90(1):119–130
Harmer CJ, Hitchcott PK, Morutto SL, Phillips GD (1997) Repeated d-amphetamine enhances stimulated mesoamygdaloid dopamine transmission. Psychopharmacology (Berl) 132(3):247–254
Hearing M, Kotecki L, Fernandez M, de Velasco E, Fajardo-Serrano A, Chung HJ, Luján R, Wickman K (2013) Repeated cocaine weakens GABA(B)-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron 80(1):159–170
Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S (2010) Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 66(6):896–907
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35(1):217–238
Kourrich S, Rothwell PE, Klug JR, Thomas MJ (2007) Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci 27(30):7921–7928
Lett BT (1989) Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 98(3):357–362
Lobo MK, Nestler EJ (2011) The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat 5:41
Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Nestler EJ (2010) Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330(6002):385–390
Luscher C, Malenka RC (2011) Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69(4):650–663
Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28(23):6046–6053
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18(3):247–291
Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE (2011) Frontal cortex and reward-guided learning and decision-making. Neuron 70(6):1054–1069
Seymour CM, Wagner JJ (2008) Simultaneous expression of cocaine-induced behavioral sensitization and conditioned place preference in individual rats. Brain Res 1213:57–68
Ungless MA, Whistler JL, Malenka RC, Bonci A (2001) Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411(6837):583–587
Volkow ND, Baler RD (2014) Addiction science: uncovering neurobiological complexity. Neuropharmacology 76(Pt B):235–249
Wheeler RA, Carelli RM (2009) Dissecting motivational circuitry to understand substance abuse. Neuropharmacology 56(Suppl 1):149–159
Wyvell CL, Berridge KC (2001) Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci 21(19):7831–7840
Acknowledgments
This work was supported by NSERC Discovery Grants 402642 and 240790 awarded to RI and SE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, D., Schumacher, A., Erb, S. et al. Aberrant approach-avoidance conflict resolution following repeated cocaine pre-exposure. Psychopharmacology 232, 3573–3583 (2015). https://doi.org/10.1007/s00213-015-4006-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4006-y