Abstract
Rationale
Low doses of psychostimulants such as methylphenidate (MPH), which increase extracellular dopamine and norepinephrine by inhibiting their reuptake, are the most commonly used treatment for attention deficit hyperactivity disorder (ADHD). Therapeutic doses of these drugs may improve focused attention at the expense of hindering other cognitive functions, including the ability to adapt behavior in response to changing circumstances—cognitive flexibility. Cognitive flexibility is thought to depend on proper operation of the prefrontal cortex (PFC) and is also linked to reward processing, which is dopamine-dependent. Additionally, reward outcome signals have been recorded from the PFC.
Objectives
This study tested the hypothesis that therapeutic doses of MPH impair cognitive flexibility and that this impairment in performance resulted from interference in reward signals within the PFC.
Methods
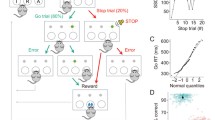
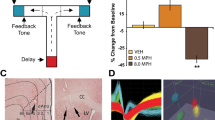
Four rhesus monkeys were given therapeutically relevant doses of oral MPH (0, 3, and 6 mg/kg) while performing an oculomotor switching task to evaluate its effect on task performance. Single-unit recordings in the PFC of two monkeys were taken before and after MPH administration during task performance.
Results
The results show that MPH does hinder switching task performance, an effect that was correlated with a reduction in the amplitude of outcome signals found in the discharges of some neurons in the PFC.
Conclusions
Methylphenidate impaired task-switching performance, which can be used as a measure of cognitive flexibility. This detriment may result from degraded outcome signaling within the PFC. This study has implications for the use of MPH in the treatment of ADHD.






Similar content being viewed by others
References
Asaad WF, Eskandar EN (2011) Encoding of both positive and negative reward prediction errors by neurons of the primate lateral prefrontal cortex and caudate nucleus. J Neurosci 31:17772–17787
Asaad WF, Rainer G, Miller EK (2000) Task-specific neural activity in the primate prefrontal cortex. J Neurophysiol 84:451–459
Bannon MJ, Roth RH (1983) Pharmacology of mesocortical dopamine neurons. Pharmacol Rev 35:53–68
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc Ser B-Stat Methodol 57:289–300
Berridge CWC, Devilbiss DMD, Andrzejewski MEM, Arnsten AFTA, Kelley AEA, Schmeichel BB, Hamilton CC, Spencer RCR (2006) Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60:1111–1120
Birn RM, Converse AK, Rajala AZ, Alexander AL, Block WF, McMillan AB, Christian BT, Filla CN, Murali D, Hurley SA, Jenison RL, Populin LC (2019) Changes in endogenous dopamine induced by methylphenidate predict functional connectivity in nonhuman primates. J Neurosci 39:1436–1444
Cools R, Clark L, Owen AM, Robbins TW (2002) Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci 22:4563–4567
Cortese A, De Martino B, Kawato M (2019) The neural and cognitive architecture for learning from a small sample. Curr Opin Neurobiol 55:133–141
Dayan P, Balleine BW (2001) Reward, motivation, and reinforcement learning. Neuron 36:285–298
Dela Pena IC, Shen GF, Shi WX (2018) Methylphenidate significantly alters the functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. Neuropharmacology 131:431–439
Dias R, Robbins TW, Roberts AC (1996) Primate analogue of the Wisconsin card sorting test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci 110:872–886
Doerge DR, Fogle CM, Paule MG, McCullagh M, Bajic S (2000) Analysis of methylphenidate and its metabolite ritalinic acid in monkey plasma by liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 14:619–623
Dyme IZ, Sahakian BJ, Golinko BE, Rabe EF (1982) Perseveration induced by methylphenidate in children: preliminary findings. Prog Neuro-Psychopharmacol Biol Psychiatry 6:269–273
Efron B, Tibshirani R (1993) An introduction to the bootstrap. Chapman & Hall, New York
Everling S, DeSouza JFX (2005) Rule-dependent activity for prosaccades and antisaccades in the primate prefrontal cortex. J Cogn Neurosci 17:1483–1496
Gamo NJ, Wang M, Arnsten AFT (2010) Methylphenidate and atomoxetine enhance prefrontal function through α2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry 49:1011–1023
Gelman A, Hill J (2007) Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, New York
Glowinski J, Tassin J, Thierry AM (1984) The mesocortico-prefrontal dopaminergic neurons. Trends Neurosci 7:415–418
Greenhill LL (2001) Clinical effects of stimulant medication in ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX (eds) Stimulant drugs and ADHD: basic and clinical neuroscience. Oxford University Press, Oxford, pp 31–71
Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacol 35:4–26
Haber SN, Lyndbalta E, Mitchell SJ (1993) The organization of the descending ventral pallidal projections in the monkey. J Comp Neurol 329:111–128
Hallett PE (1978) Primary and secondary saccades to goals defined by instructions. Vis Res 18:1279–1296
Hornak J, O'Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE (2004) Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci 16:463–478
Judge SJ, Richmond BJ, Chu C (1980) Implantation of magnetic search coils for measurement of eye position: an improved method. Vis Res 20:535–538
Kennerley SW, Wallis JD (2009) Evaluating choices by single neurons in the frontal lobe: outcome value encoded across multiple decision variables. Eur J Neurosci 29:2061–2073
Kodama T, Kojima T, Honda Y, Hosokawa T, Tsutsui K-I, Watanabe M (2017) Oral administration of methylphenidate (Ritalin) affects dopamine release differentially between the prefrontal cortex and striatum: a microdialysis study in the monkey. J Neurosci 37:2387–2394
Lewis BL, O'Donnell P (2000) Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D-1 dopamine receptors. Cereb Cortex 10:1168–1175
MacPherson JM, Aldridge JW (1979) A quantitative method of computer analysis of spike train data collected from behaving animals. Brain Res 175:183–187
Mansouri FA, Matsumoto K, Tanaka K (2006) Prefrontal cell activities related to monkeys’ success and failure in adapting to rule changes in a Wisconsin card sorting test analog. J Neurosci 26:2745–2756
Marcos E, Nougaret S, Tsujimoto S, Genovesio A (2018) Outcome modulation across tasks in the primate dorsolateral prefrontal cortex. Neurosci 371:96–105
Matsumoto M, Matsumoto K, Abe H, Tanaka K (2007) Medial prefrontal cell activity signaling prediction errors of action values. Nat Neurosci 10:647–656
Milner B (1963) Effects of different brain lesions on card sorting: the role of the frontal lobes. Arch Neurol 9:90–100
Monchi O, Petrides M, Petre V, Worsley K, Dagher A (2001) Wisconsin card sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci 21:7733–7741
Nakahara K, Hayashi T, Konishi S, Miyashita Y (2002) Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science 295:1532–1536
Niki H, Watanabe M (1979) Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res 171:213–224
Oemisch M, Westendorff S, Azimi M, Hassani SA, Ardid S, Tiesinga P, Womelsdorf T (2019) Feature-specific prediction errors and surprise across macaque fronto-striatal circuits. Nat Commun 10:1–15
Parent A, Hazrati LN (1995) Functional-anatomy of the basal ganglia. 1. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev 20:91–127
Rajala AZ, Henriques JB, Populin LC (2012) Dissociative effects of methylphenidate in nonhuman primates: trade-offs between cognitive and behavioral performance. J Cogn Neurosci 24:1371–1381
Rajala AZ, Yan Y, Dent ML, Populin LC (2013) The inferior colliculus encodes the Franssen auditory spatial illusion. Eur J Neurosci 38:3056–3070
Rajala AZ, Jenison RL, Populin LC (2018) Neural correlate of auditory spatial attention allocation in the superior colliculus. J Neurophysiol 119:1450–1460
Richmond BJ, Optican LM (1987) Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. II Quantification of response waveform. J Neurophysiol 57:147–161
Robbins TW, Sahakian BJ (1979) “Paradoxical” effects of psychomotor stimulant drugs in hyperactive children from the standpoint of behavioural pharmacology. Neuropharmacol 18:931–950
Robinson DA (1963) A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10:137–145
Rolls ET, Thorpe SJ, Boytim M, Szabo I, Perrett DI (1984) Responses of striatal neurons in the behaving monkey. 3. Effects of iontophoretically applied dopamine on normal responsiveness. Neurosci 12:1201–1212
Schultz W, Apicella P, Ljungberg T (1993) Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci 13:900–913
Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275:1593–1599
Solanto MV, Wender EH (1989) Does methylphenidate constrict cognitive functioning? J Am Acad Child Adolesc Psychiatry 28:897–902
Tannock R, Schachar R (1992) Methylphenidate and cognitive perseveration in hyperactive children. J Child Psychol Psychiatry 33:1217–1228
Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ (2002) Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studes. Eur Neuropsychopharmacol 12:557–566
Wallis JD, Anderson KC, Miller EK (2001) Single neurons in prefrontal cortex encode abstract rules. Nature 411:953–956
Watanabe M (1989) The appropriateness of behavioral responses coded in post-trial activity of primate prefrontal units. Neurosci Lett 101:113–117
White IM, Wise SP (1999) Rule-dependent neuronal activity in the prefrontal cortex. Exp Brain Res 126:315–335
Williams SM, Goldman-Rakic PS (1993) Characterization of the dopaminergic innervation of the primate frontal cortex using a dopamine specific antibody. Cereb Cortex 3:199–222
Wise S (2008) Forward frontal fields: phylogeny and fundamental function. Trends Neurosci 31:599–608
Acknowledgements
We thank the Animal Care Staff of the UW School of Medicine and Public Health and the Wisconsin National Primate Research Center. We also thank Katharine Reininger and Kimberly Lancaster for help with data collection and Yonghe Yan for computer programming.
Funding
This work was supported by grants from the National Institutes of Health (AA018736, DC003693), and the National Science Foundation (IOB-051458).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 433 kb)
Rights and permissions
About this article
Cite this article
Rajala, A.Z., Populin, L.C. & Jenison, R.L. Methylphenidate affects task-switching and neural signaling in non-human primates. Psychopharmacology 237, 1533–1543 (2020). https://doi.org/10.1007/s00213-020-05478-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05478-z