Abstract
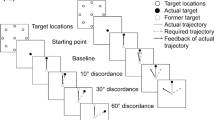
We investigated whether deficits of adaptive improvement in seniors are related to an age-dependent decay of the brain’s executive functions. Younger and older subjects completed a battery of cognitive tests, and preformed aimed arm movements before and during exposure to rotated visual feedback. In accordance with previous work, we found that adaptive improvement during exposure was degraded in seniors, while the transfer of adaptation to a new motor task was not. This pattern of findings confirms that strategic control but not sensorimotor recalibration is affected by old age. Using multiple linear regression (MLR) to extract separate executive components from our test battery, we found that basic response speed and decision-making, but not the inhibition of prepotent responses or mental flexibility, were degraded in our older subjects. Again using MLR, we found that degraded adaptive improvement in our seniors was partly related to the decay of basic response speed and decision-making, and partly to age-dependent phenomena not addressed by our cognitive-test battery. Finally, we observed that interindividual variability of cognition and adaptive improvement was larger in old than in young subjects, which could explain why some previous studies found degraded adaptation in seniorswhile others did not.



Similar content being viewed by others
References
Bock O (2005) Components of sensorimotor adaptation in young and elderly subjects. Exp Brain Res 160:259–263
Bock O, Schneider S (2001) Acquisition of a sensorimotor skill in younger and older adults. Acta Physiol Pharmacol Bulg 26:89–92
Brown S (1996) Control of simple arm movements in the elderly. In: Ferrandez A-M, Teasdale N (eds) Changes in sensory motor behavior in aging. Elsevier, Amsterdam, pp 27–52
Buch E, Young S, Contreras-Vidal J (2003) Visuomotor adaptation in normal aging. Learn Mem 10:55–63
Canavan AGM, Bayer N, Lutzenberger W (eds) (1993) The decline of spatial and temporal abilities with age. Kluwer, Dordrecht
Cerella J (1985) Information processing rates in the elderly. Psychol Bull 98:67–83
Crossman E, Szafran J (1956) Changes with age in the speed of information intake and discrimination. Experientia 4(Suppl):128–135
Etnier JL, Landers DM (1998) Motor performance and motor learning as a function of age and fitness. R Q Exerc Sports 69:136–146
Fernández-Ruiz J, Hall C, Vergara P, Díaz R (2000) Prism adaptation in normal aging: slower adaptation rate and larger aftereffect. Cogn Brain Res 9:223–226
Houx P, Jolles J, Vreeling F (1993) Stroop interference: aging effects assessed with the stroop color-Word test. Exp Aging Res 19:209–224
Ingram H, van Donkelaar P, Cole J, Vercher J-L, Gauthier G, Miall R (2000) The role of proprioception and attention in a visuomotor adaptation task. Exp Brain Res 132:114–126
Kagerer F, Contreras-Vidal J, Stelmach G (1997) Adaption to gradual as compared with sudden visuo-motor distortions. Exp Brain Res 115:557–561
McNay EC, Willingham DB (1998) Deficit in learning of a motor skill requiring strategy, but not of perceptualmotor recalibration, with aging. Learn Mem 4:411–420
Raz N (2000) Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik F, Salthouse T (eds) The handbook of aging and cognition. Erlbaum, Hillsdale, NJ, pp1–90
Redding G (1996) Adaptive spatial alignment and strategic perceptual-motor control. J Exp Psychol: Hum Percept Perform 22:379–394
Rhodes M (2004) Age-related differences in performance on the Wisconsin card sorting test: a meta-analytic review. Psychol Aging 19:482–494
Ridderinkhof K, Span M, van der Molen M (2002) Perseverative behavior and adaptive control in older adults: performance monitoring, rule induction, and set shifting. Brain Cogn 49:382–401
Roller C, Cohen H, Kimball K, Bloomberg J (2002) Effects of normal aging on visuo-motor plasticity. Neurobiol Aging 23:117–123
Schaie K (1988) Variability in cognitive function in the elderly: implications for societal participation. In: Woodhead A, Bender M, Leonard R (eds) Phenotypic variation in populations: relevance to risk assessment. Plenum Press, NY, pp 191–211
Spirduso W (1995) Physical dimensions of aging Human kinetics. University of Illinois Press, Champaign IL
Stratton GM (1897) Vision without inversion of the retinal image. Psychol Rev 4:341–481
Stuss D, Benson D (1986) The frontal lobes. Raven Press, NY
Suzi G, Davidoff M, Surwillo W (1960) Reaction time as a function of stimulus information and age. J Exp Psychol 60:242–244
Volkow N, Gur R, Gwang G-J, Fowler J, Moberg P, Ding Y-S, Hitzemann R, Smith G, Logan J (1998) Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiat 155:344–349
West R, Alain C (2000) Age-related decline in inhibitory control contributes to the increased Stroop effect observed in older adults. Psychophysiology 37:179–189
Wright B, Payne R (1985) Effects of aging on sex differences in psychomotor reminiscence and tracking proficiency. J Gerontol 40:179–184
Acknowledgments
This work was supported by DFG grant BO 649/8. Thanks are due to Dipl.-Ing. (FH) Lutz Geisen for software development, and to Ms. Laura Walk for assistance with data analysis.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Partitioning Of Variances
Consider the linear equation:
where x k and y k are performance measures from two different behavioral tests, x and z. The total variance of y can be partitioned into several components. The variance which y shares with any of x k can be quantified as the squared multiple correlation between y and all x k , denoted as R 2(x 1, x 2, x 3), or for short as R 2(x). If R 2(x) is significant, then test x makes a reliable contribution to y.
In Fig. 4, the total variance of x k is represented by the circle x, which can be thought of as the envelope of three shapes representing x 1, x 2, and x 3, respectively. Area a+b, therefore, reflects the variance shared between y and any of x k , or R 2(x). Similarly, area a+b+c represents the variance shared between y and any of x k and z k , quantified as R 2(x 1, x 2, x 3, z 1, z 2, z 3), or for short as R 2(x, z).
The concept of common and unique variance, as the basis of MLR analyses. The top circle represents the variance of an independent variable y, and the bottom circles the variance of two groups of independent variables x and z. The overlap reflects the variance of y shared with x only (area a), z only (area c), and with x and z jointly (area b)
Based on these considerations we can determine area c, which represents the variance shared between y and z but not x, as R 2(x, z) - R 2(x); if this term is significant, then test z makes a reliable contribution to y which cannot be explained by test x. We can also determine area b, which represents the variance shared between y and both tests jointly, as R 2(x) + R 2(z) - R 2(x, z). If this value is significant, then both tests make a joint contribution to y.
To consider a third test w, we can combine tests x and z and then re-apply the above reasoning. For example, the variance shared between y and w but not x nor z can be calculated as R 2(x, y, z) - R 2(x, y).
Rights and permissions
About this article
Cite this article
Bock, O., Girgenrath, M. Relationship between sensorimotor adaptation and cognitive functions in younger and older subjects. Exp Brain Res 169, 400–406 (2006). https://doi.org/10.1007/s00221-005-0153-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0153-4