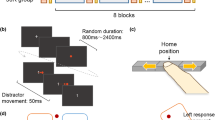
Abstract
Observers can learn to move in novel, adapted environments after watching a learning or expert model. Although this is an effective practice technique, it is unclear how this learning is achieved and if observers update an internal model of their visual–motor environment, as shown through the presence of after-effects (i.e., negative carry-over effects when aiming in a normal environment following exposure to perturbed conditions). For such updating to occur via observational practice, it has been reasoned that the observer requires the motor capabilities to perform the task they are observing. To test this, we first trained three groups to physically move in clockwise (CW) or counterclockwise (CCW) rotated environments. When immediately returned to a normal environment, after-effects were seen. We then attempted to wash out these effects before allowing two of these groups (CW and CCW), and a naïve observation only group, to watch a video of an actor performing in a CW environment. This observation phase was immediately followed by another test for after-effects and a direct test of learning when aiming in the rotated environment. Consistent with previous data, there were direct learning effects due to observation. Although after-effects increased for the experienced observers, these were small and were not significantly different from a physical practice only group that did not undergo the observation phase. Therefore, even with a motor repertoire for the rotated environment, there was a lack of evidence that observational practice results in implicit (re)updating of an internal model for aiming.


Similar content being viewed by others
Notes
There were originally n = 12 participants in the ActCCW + Obs group, but during the analysis stage one individual was removed due to unusually low errors in practice and subsequent knowledge that they had participated in a previous, similar experiment. We tested a couple more participants in our primary condition (ActCW + Obs) to allow greater confidence in our conclusions.
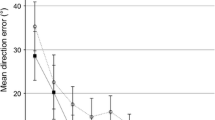
We also looked at after-effects on a smaller time scale (i.e., a cycle of 5 trials, rather than a 25 trial block), to see if potential after-effects dissipated quickly. There was no evidence that this was the case with after-effects remaining relatively consistent within a 25-trial block. We ran a 4 Group × 2 Block × 5 Cycle Repeated Measures ANOVA. None of the effects involving cycle were significant (F < 1 for the cycle main effect).
Based on a Reviewer’s suggestion, we considered the possibility that after-effects in Phase II might be reduced for a group who had two phases of CW physical practice, potentially as a result of the additional blocks of washout interspersed before the second test for after-effects. This raises the possibility that the small after-effects we saw from our previous ActCW groups (ActCW + Obs; ActCW_only) would have been observed even after a second physical practice phase. Testing of six individuals ActCW + ActCW who underwent the same washout procedures showed this not to be the case. After-effects following a second phase of physical practice were M = −20.88° (Block 1) and M = −15.49° (Block 2), similar to the size of the after-effects after the first physical practice block (Block 1, M = −20.40 and Block 2, M = −14.65°). These values were of a significantly greater magnitude than those seen by the groups who had only one phase of CW physical practice and they were also commensurate with the Posttest1 data from these two groups (ActCW_only and ActCW + Obs).
References
Ashford D, Davids K, Bennett SJ (2007) Observational modeling effects for movement dynamics and movement outcome 5 measures across differing task constraints: a meta-analysis. J Mot Behav 38:185–205
Bandura A (1971) Analysis of modeling processes. In: Bandura A (ed) Psychological modeling: conflicting theories. Adline-Atherton, Chicago, pp 1–62
Bandura A (1986) Social foundations of thought and action: a social cognitive theory. Prentice Hall, Englewood Cliffs
Benson BL, Anguera JA, Seidler RD (2011) A spatial strategy reduces error but interferes with sensorimotor adaptation. J Neurophys 105:2843–2851
Bird G, Heyes C (2005) Effector-dependent learning by observation of a finger movement sequence. J Exp Psychol Hum Percept Perform 31:262–275
Brown LE, Wilson ET, Gribble PL (2009) Repetitive transcranial magnetic stimulation to the primary motor cortex interferes with motor learning by observing. J Cog Neurosci 21(5):1013–1022. doi:10.1162/jocn.2009.21079
Calvo-Merino B, Glaser DE, Grezes J, Passingham RE, Haggard P (2005) Action observation and acquired motor skills: an fMRI study with expert dancers. Cereb Cortex 15(8):1243–1249. doi:10.1093/cercor/bhi007
Calvo-Merino B, Grezes J, Glaser DE, Passingham RE, Haggard P (2006) Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol 16(19):1905–1910. doi:10.1016/j.cub.2006.07.065
Caspers S, Zilles K, Laird AR, Eickhoff SB (2010) ALE meta-analysis of action observation and imitation in the human brain. NeuroImage 50:1148–1167
Cross ES, Hamilton A, Grafton ST (2006) Building a motor simulation de novo: observation of dance by dancers. NeuroImage 31:1257–1267
Deakin JM, Proteau L (2000) The role of scheduling in learning through observation. J Mot Behav 32(3):268–276
Decety J, Grèzes J, Costes N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F (1997) Brain activity during observation of actions. Influence of action content and subject’s strategy. Brain 120:1763–1777
Demougeot L, Papaxanthis C (2011) Muscle fatigue affects mental simulation of action. J Neurosci 31(29):10712–10720. doi:10.1523/jneurosci.6032-10.2011
Fadiga L, Craighero L (2004) Electrophysiology of action representation. J Clin Neurophysiol 21(3):157–169
Fernandez-Ruiz J, Wong W, Armstrong IT, Flanagan J (2011) Relation between reaction time and reach errors during visuomotor adaptation. Behav Brain Res 219:8–14
Gentile AM (1998) Implicit and explicit processes during acquisition of functional skills. Scand J Occup Ther 5:7–16
Gentili R, Cahouet V, Ballay Y, Papaxanthis C (2004) Inertial properties of the arm are accurately predicted during motor imagery. Behav Brain Res 155(2):231–239. doi:10.1016/j.bbr.2004.04.027
Grèzes J, Decety J (2001) Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum Brain Mapp 12:1–19. doi:10.1002/1097-0193(200101)12:1<1:AID-HBM10>3.0.CO;2-V
Gruetzmacher N, Panzer S, Blandin Y, Shea CH (2011) Observation and physical practice: coding of simple motor sequences. Q J Exp Psychol 64(6):1111–1123. doi:10.1080/17470218.2010.543286
Hatada Y, Miall RC, Rossetti Y (2006) Two waves of a long-lasting after effect of prism adaptation measured over 7 days. Exp Brain Res 169:417–426
Held R (1965) Plasticity in sensory-motor systems. Sci Am 213:84–94
Henriques DYP, Cressman EK (2012) Visuomotor adaptation and proprioceptive recalibration. J Motor Behav 44:435–444
Higuchi S, Holle H, Roberts N, Eickhoff SB, Vogt S (2012) Imitation and observational learning of hand actions: prefrontal involvement and connectivity. NeuroImage 59:1668–1683
Hinder MR, Walk L, Woolley DG, Riek S, Carson RG (2007) The interference effects of non-rotated versus counter-rotated trials in visuomotor adaptation. Exp Brain Res 180:629–640
Hinder MR, Riek S, Tresilian JR, De Rugy A, Carson RG (2010) Real-time error detection but not error correction drives automatic visuomotor adaptation. Exp Brain Res 201:191–207
Hodges NJ, Franks IM (2001) Learning a coordination skill: interactive effects of instruction and feedback. Res Q Exer Sport 72:132–142
Iacoboni M (2005) Neural mechanisms of imitation. Curr Opin Neurobiol 15:632–637
Ingram HA, van Donkelaar P, Cole J, Vercher J-L, Gauthier GM, Miall RC (2000) The role of proprioception and attention in a visuomotor adaptation task. Exp Brain Res 132:114–126
Jeannerod M (2001) Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage 14(1 Pt 2):S103–S109. doi:10.1006/nimg.2001.0832
Kagerer FA, Contreras-Vidal JL, Stelmach GE (1997) Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res 115:557–561
Kelly SW, Burton AM, Riedel B, Lynch E (2003) Sequence learning by action and observation: evidence for separate mechanisms. Br J Psychol 94:355–372. doi:10.1348/000712603767876271
Klapp ST, Nordell SA, Hoekenga KC, Patton CB (1974) Long-lasting aftereffect of brief prism exposure. Percep Psychophys 15:399–400
Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G (2002) Hearing sounds, understanding actions: action representation in mirror neurons. Science 297:846–848
Krakauer JW (2009) Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol 629:405–521. doi:10.1007/978-0-387-77064-2_21
Larssen BC, Ong NT, Hodges NJ (2012) Watch and learn: seeing is better than doing when acquiring consecutive motor tasks. PLoS ONE 7(6):e38938. doi:10.1371/journal.pone.0038938
Malfait N, Valyear KF, Culham JC, Anton JL, Brown LE, Gribble PL (2010) fMRI activation during observation of others’ reach errors. J Cog Neurosci 22:1493–1503
Maslovat D, Hodges NJ, Krigolson OE, Handy TC (2010) Observational practice benefits are limited to perceptual improvements in the acquisition of a novel coordination skill. Exp Brain Res 204:119–130. doi:10.1007/s00221-010-2302-7
Maslovat D, Chua R, Hodges NJ (2013) When unintended movements “leak” out: a startling acoustic stimulus can elicit a prepared response during motor imagery and action observation. Neuropsychologia 5:838–844. doi:10.1016/j.neuropsychologia.2013.01.016
Mattar AA, Gribble PL (2005) Motor learning by observing. Neuron 46:153–160. doi:10.1016/j.neuron.2005.02.009
Mazzoni P, Krakauer JW (2006) An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26:3642–3645
Munzert J, Zentgraf K, Stark R, Vaitl D (2008) Neural activation in cognitive motor processes: comparing motor imagery and observation of gymnastic movements. Exp Brain Res 188:437–444. doi:10.1007/s00221-008-1376-y
Ong NT, Hodges NJ (2010) Absence of after-effects for observers after watching a visuomotor adaptation. Exp Brain Res 205:325–334. doi:10.1007/s00221-010-2366-4
Ong NT, Larssen BC, Hodges NJ (2012) In the absence of physical practice, observation and imagery do not result in updating of internal models for aiming. Exp Brain Res 218:9–19. doi:10.1007/s00221-011-2996-1
Osman M, Bird G, Heyes C (2005) Action observation supports effector-dependent learning of finger movement sequences. Exp Brain Res 165:19–27. doi:10.1007/s00221-005-2275-0
Rizzolatti G, Sinigaglia C (2010) The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci 11:264–274
Rizzolatti G, Fogassi L, Gallese V (2001) Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Neurosci 2:661–670
Ronchi R, Revol P, Katayama M, Rossetti Y, Farnè A (2011) Seeing your error alters my pointing: observing systematic pointing errors induces sensori-motor after-effects. PLoS ONE 6:e21070. doi:10.1371/journal.pone.0021070
Rumiati RI, Weiss PH, Tessari A, Assmus A, Zilles K, Herzog H, Fink GR (2005) Common and differential neural mechanisms supporting imitation of meaningful and meaningless actions. J Cog Neurosci 17:1420–1431
Salomonczyk D, Cressman EK, Henriques DY (2011) Proprioceptive recalibration following prolonged training and increasing distortions in visuomotor adaptation. Neuropsych 49:3053–3362
Shadmehr R, Mussa-Ivaldi FA (1994) Adaptive representation of dynamics during learning a motor task. J Neurosci 14:3208–3224
Taylor JA, Ivry RB (2011) Flexible cognitive strategies during motor learning. PLoS Comp Bio 7(2):e1001096
Umilta MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G (2001) I know what you are doing: a neurophysiological study. Neuron 31:155–165
Vogt S (2002) Visuomotor couplings in object-oriented and imitative actions. In: Meltzoff AN, Prinz W (eds) The imitative mind: development, evolution, and brain bases. Cambridge University Press, Cambridge, pp 206–220
Vogt S, Thomaschke R (2007) From visuo-motor interactions to imitation learning: behavioural and brain imaging studies. J Sports Sci 25:3–23
Vogt S, Buccino G, Wohlschläger AM, Canessa N, Shah NJ, Zilles K, Eickhoff SB, Freund H-J, Rizzolatti G, Fink GR (2007) Prefrontal involvement in imitation learning of hand actions: effects of practice and expertise. NeuroImage 37:1371–1383
Williams A, Gribble PL (2012) Observed effector-independent motor learning by observing. J Neurophys 107:1564–1570
Wolpert DM, Flanagan JR (2001) Motor prediction. Curr Biol 11(18):R729–R732. doi:10.1016/S0960-9822(01)00432-8
Wolpert DM, Miall RC (1996) Forward models for physiological motor control. Neural Net 9:1265–1279. doi:10.1016/S0893-6080(96)00035-4
Wolpert DM, Diedrichsen J, Flanagan JR (2011) Principles of sensorimotor learning. Nat Rev Neurosci 12:739–751
Acknowledgments
This research was supported by a Discovery Grant to the final author from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, S.B., Larssen, B.C. & Hodges, N.J. Manipulating visual–motor experience to probe for observation-induced after-effects in adaptation learning. Exp Brain Res 232, 789–802 (2014). https://doi.org/10.1007/s00221-013-3788-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-013-3788-6