Abstract
Introduction
Neuromelanin-sensitive MRI has been reported to be used in the diagnosis of Parkinson’s disease (PD), which results from loss of dopamine-producing cells in the substantia nigra pars compacta (SNc). In this study, we aimed to apply a 3D turbo field echo (TFE) sequence for neuromelanin-sensitive MRI and to evaluate the diagnostic performance of semi-automated method for measurement of SNc volume in patients with PD.
Methods
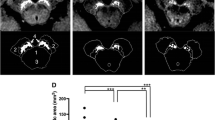
We examined 18 PD patients and 27 healthy volunteers (control subjects). A 3D TFE technique with off-resonance magnetization transfer pulse was used for neuromelanin-sensitive MRI on a 3T scanner. The SNc volume was semi-automatically measured using a region-growing technique at various thresholds (ranging from 1.66 to 2.48), with the signals measured relative to that for the superior cerebellar peduncle. Receiver operating characteristic (ROC) analysis was performed at all thresholds. Intra-rater reproducibility was evaluated by intraclass correlation coefficient (ICC).
Results
The average SNc volume in the PD group was significantly smaller than that in the control group at all the thresholds (P < 0.01, student t test). At higher thresholds (>2.0), the area under the curve of ROC (Az) increased (0.88). In addition, we observed balanced sensitivity and specificity (0.83 and 0.85, respectively). At lower thresholds, sensitivity tended to increase but specificity reduced in comparison with that at higher thresholds. ICC was larger than 0.9 when the threshold was over 1.86.
Conclusions
Our method can distinguish the PD group from the control group with high sensitivity and specificity, especially for early stage of PD.





Similar content being viewed by others
References
Greenfield JG, Bosanquet FD (1953) The brain-stem lesions in Parkinsonism. J Neurol Neurosurg Psychiat 16:213–226
Fedorow H, Tribl F, Halliday G, Gerlach M, Riederer P, Double KL (2005) Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson’s disease. Prog Neurobiol 75:109–124
Enochs WS, Petherick P, Bogdanova A, Mohr U, Weissleder R (1997) Paramagnetic metal scavenging by melanin: MR imaging. Radiology 204:417–423
Hughes AJ, Daniel SE, Kilford L, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Orimo S, Ozawa E, Nakade S, Sugimoto T, Mizusawa H (1999) 123I-metaiodobenzylguanidine myocardial scintigraphy in Parkinson’s disease. J Neurol Neurosurg Psychiat 67:189–194
Sasaki M, Shibata E, Kudo K, Tohyama K (2008) Neuromelanin-sensitive MRI. Clin Neuroradiol 18:147–153
Sasaki M, Shibata E, Tohyama K, Takahashi J, Otsuka K, Tsuchiya K, Takahashi S, Ehara S, Terayama Y, Sakai A (2006) Neuromelanin magnetic resonance imaging of lucus ceruleus and substantia nigra in Parkinson’s disease. Neuroreport 17:1215–1218
Shibata E, Sasaki M, Tohyama K, Otsuka K, Endoh J, Terayama Y, Sasaki A (2008) Use of neuromelanin-sensitive MRI to distinguish schizophrenic and depressive patients and healthy individuals based on signal alterations in the substantia nigra and locus ceruleus. Biol Psychiatry 64:401–406
Sasaki M, Inoue T, Tohyama K, Oikawa H, Ehara S, Ogawa A (2003) High-field MRI of the central nervous system: current approaches to clinical and microscopic imaging. Magn Reson Med Sci 2:133–139
Truong TK, Chakeres DW, Beversdorf DQ, Scharre DW, Schmalbrock P (2006) Effects of static and rediofrequency magnetic field inhomogeneity in ultra-high field magnetic resonance imaging. Magn Reson Imaging 24:103–112
Kudo K, Sasaki M, Yamada K, Momoshima S, Utsunomiya H, Shirato H, Ogasawara K (2010) Differences in CT perfusion maps generated by different commercial software: quantitative analysis by using identical source data of acute stroke patients. Radiology 254(1):200–209
Nakane N, Nihashi T, Kawai H, Naganawa S (2008) Visualization of neuromelanin in the substantia nigra and locus ceruleus at 1.5T using a 3D-gradient echo sequence with magnetization transfer contrast. Magn Reson Med Sci 7(4):205–210
Fearnley JM, Lees AJ (1991) Aging and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(5):2283–2301
Kashihara K, Shinya T, Higaki F (2011) Neuromelanin magnetic resonance imaging of nigral volume loss in patients with Parkinson’s disease. J Clin Neurosci 18:1093–1096
Menke RA, Scholz J, Miller KL, Deoni S, Jbabdi S, Matthews PM, Zarei M (2009) MRI characteristics of the substantia nigra in Parkinson’s disease: a combined quantitative T1 and DTI study. NeuroImage 47(2):435–441
Wang JJ, Lin WY, Lu CS, Weng YH, Ng SH, Wang CH, Liu HL, Hsieh RH, Wan YL, Wai YY (2011) Parkinson disease: diagnostic utility of diffusion kurtosis imaging. Radiology 261(1):210–217
Wallis LI, Paley MNJ, Graham JM, Grünewald RA, Wignall EL, Joy HM, Griffiths PD (2008) MRI assessment of basal ganglia iron deposition in Parkinson’s disease. J Magn Reson Imaging 28(5):1061–1067
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogisu, K., Kudo, K., Sasaki, M. et al. 3D neuromelanin-sensitive magnetic resonance imaging with semi-automated volume measurement of the substantia nigra pars compacta for diagnosis of Parkinson’s disease. Neuroradiology 55, 719–724 (2013). https://doi.org/10.1007/s00234-013-1171-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-013-1171-8