Abstract
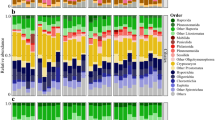
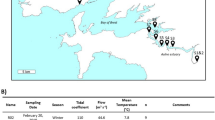
Seasonal changes in environmental conditions have a strong impact on microbial community structure and dynamics in aquatic habitats. To better elucidate the response of bacterial communities to environmental changes, we have measured a large variety of limnetic variables and investigated bacterial community composition (BCC) and dynamics over seven consecutive years between 2003 and 2009 in mesotrophic Lake Tiefwaren (NE Germany). We separated between free-living (FL, >0.2, <5.0 μm) and particle-associated (PA, >5.0 μm) bacteria to account for different bacterial lifestyles and to obtain a higher resolution of the microbial diversity. Changes in BCC were studied by DGGE based on PCR-amplified 16S rRNA gene fragments. Sequencing of DGGE bands revealed that ca. 70 % of all FL bacteria belonged to the Actinobacteria, whereas PA bacteria were dominated by Cyanobacteria (43 %). FL communities were generally less diverse and rather stable over time compared to their PA counterpart. Annual changes in reoccurring seasonal patterns of dominant freshwater bacteria were supported by statistical analyses, which revealed several significant correlations between DGGE profiles and various environmental variables, e.g. temperature and nutrients. Overall, FL bacteria were generally less affected by environmental changes than members of the PA fraction. Close association of PA bacteria with phytoplankton and zooplankton suggests a tight coupling of PA bacteria to organisms of higher trophic levels. Our results indicate substantial differences in bacterial lifestyle of pelagic freshwater bacteria, which are reflected by contrasting seasonal dynamics and relationships to a number of environmental variables.



Similar content being viewed by others
References
Fuhrman JA, Hewson I, Schwalbach MS, Steele JA, Brown MV, Naeem S (2006) Annually reoccurring bacterial communities are predictable from ocean conditions. Proc Natl Acad Sci U S A 103:13104–13109
Post AF, Penno S, Zandbank K, Paytan A, Huse SM, Welch DM (2011) Long term seasonal dynamics of Synechococcus population structure in the Gulf of Aqaba, Northern Red Sea. Front Microbiol 2:Article 131, doi:110.3389/fmicb.2011.00131
Schauer M, Balagué V, Pedrós-Alió C, Massana R (2003) Seasonal changes in the taxonomic composition of bacterioplankton in a coastal oligotrophic system. Aquat Microb Ecol 31:163–174
Crump BC, Hobbie JE (2005) Synchrony and seasonality in bacterioplankton communities of two temperate rivers. Limnol Oceanogr 50:1718–1729
Shade A, Kent AD, Jones SE, Newton RJ, Triplett EW, McMahon KD (2007) Interannual dynamics and phenology of bacterial communities in a eutrophic lake. Limnol Oceanogr 52:487–494
Fuhrman JA, Steele JA (2008) Community structure of marine bacterioplankton: patterns, networks, and relationships to function. Aquat Microb Ecol 53:69–81
Van der Gucht K, Sabbe K, De Meester L, Vloemans N, Zwart G, Gillis M, Vyverman W (2001) Contrasting bacterioplankton community composition and seasonal dynamics in two neighbouring hypertrophic freshwater lakes. Environ Microbiol 3:680–690
Yannarell AC, Kent AD, Lauster GH, Kratz TK, Triplett EW (2003) Temporal patterns in bacterial communities in three temperate lakes of different trophic status. Microb Ecol 46:391–405
Allgaier M, Grossart H-P (2006) Diversity and seasonal dynamics of Actinobacteria populations in four lakes in northeastern Germany. Appl Environ Microbiol 72:3489–3497
Salcher MM, Pernthaler J, Posch T (2010) Spatiotemporal distribution and activity patterns of bacteria from three phylogenetic groups in an oligomesotrophic lake. Limnol Oceanogr 55:846–856
Salcher MM, Pernthaler J, Zeder M, Psenner R, Posch T (2008) Spatio-temporal niche separation of planktonic Betaproteobacteria in an oligo-mesotrophic lake. Environ Microbiol 10:2074–2086
Wu QL, Hahn MW (2006) High predictability of the seasonal dynamics of a species-like Polynucleobacter population in a freshwater lake. Environ Microbiol 8:1660–1666
Zeder M, Peter S, Shabarova T, Pernthaler J (2009) A small population of planktonic Flavobacteria with disproportionally high growth during the spring phytoplankton bloom in a prealpine lake. Environ Microbiol 11:2676–2686
Koschel R, Casper P, Gonsiorczyk T, Roßberg R, Wauer G (2006) Hypolimnetic Al and CaCO3 treatments and aeration for restoration of a stratified eutrophic hardwater lake in Germany. Verh Internat Verein Limnol 29:2165–2171
Mehner T, Diekmann M, Gonsiorczyk T, Kasprzak P, Koschel R, Krienitz L, Rumpf M, Schulz M, Wauer G (2008) Rapid recovery from eutrophication of a stratified lake by disruption of internal nutrient load. Ecosystems 11:1142–1156
Wauer G, Gonsiorczyk T, Hupfer M, Koschel R (2009) Phosphorus balance of Lake Tiefwarensee during and after restoration by hypolimnetic treatment with aluminum and calcium salts. Lake Reserv Manag 25:377–388
Grasshoff K, Kremling K, Ehrhadt M (eds) (1999) Methods of seawater analysis, 3rd, completely revised and extended ed., Weinheim: New York, Chiester, Brisbane, Singapore, Toronto, Wiley-VCH, Ch
Wetzel RG, Likens GE (1991) Limnological analysis, 2nd. Springer-Verlag, New York
Koroleff F (1976) Determination of phosphorus. In: Grasshoff K (ed) Methods in seawater analysis. Verlag Chemie, Weinheim, New York, pp 125–131
Hepperle D, Schmidt-Halewicz SE (2000) Opticount©. A software tool for the enumeration and biomass determination of plankton organisms and other particles
Haney J, Hall D (1973) Sugar-coated Daphnia: a preservation technique for Cladocera. Limnol Oceanogr 18:331–333
Simon M, Azam F (1989) Protein content and protein synthesis rates of planktonic marine bacteria. Mar Ecol Prog Ser 51:201–213
Kirchman, D. L (2001) Measuring bacterial biomass production and growth rates from leucine incorporation in natural aquatic environments. Methods Microbiol 30:227–237
Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Pernthaler A, Pernthaler J, Amann R (2002) Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl Environ Microbiol 68:3094–3101
Sekar R, Pernthaler A, Pernthaler J, Warnecke F, Posch T, Amann R (2003) An improved protocol for quantification of freshwater Actinobacteria by fluorescence in situ hybridization. Appl Environ Microbiol 69:2928–2935
Amann RI, Krumholz L, Stahl DA (1990) Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol 172:762–770
Daims H, Brühl A, Amann R, Schleifer KH, Wagner M (1999) The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22:434–444
Roller C, Wagner M, Amann R, Ludwig W, Schleifer KH (1994) In situ probing of Gram-positive bacteria with high DNA G + C content using 23S rRNA-targeted oligonucleotides. Microbiol 140:2849–2858
Glöckner FO, Zaichikov E, Belkova N, Denissova L, Pernthaler J, Pernthaler A, Amann RI (2000) Comparative 16S rRNA analysis of lake bacterioplankton reveals globally distributed phylogenetic clusters including an abundant group of Actinobacteria. Appl Environ Microbiol 66:5053–5065
Warnecke F, Sommaruga R, Sekar R, Hofer JS, Pernthaler J (2005) Abundances, identity, and growth state of Actinobacteria in mountain lakes of different UV transparency. Appl Environ Microbiol 71:5551–5559
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Allgaier M, Grossart H-P (2006) Seasonal dynamics and phylogenetic diversity of free-living and particle-associated bacterial communities in four lakes in northeastern Germany. Aquat Microb Ecol 45:115–128
DeSantis TZ, Hugenholtz P, Keller K, Brodie EL, Larsen N, Piceno YM, Phan R, Andersen GL (2006) NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res 34:W394–W399
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar BA, Lai T, Steppi S et al (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P et al (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S (2011) A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75:14–49
Morris DP, Lewis WM Jr (1992) Nutrient limitation of bacterioplankton growth in Lake Dillon, Colorado. Limnol Oceanogr 37:1179–1192
Newton RJ, McMahon KD (2011) Seasonal differences in bacterial community composition following nutrient additions in a eutrophic lake. Environ Microbiol 13:887–899
Cotner JB, Biddanda BA (2002) Small players, large role: microbial influence on biogeochemical processes in pelagic aquatic ecosystems. Ecosystems 5:105–121
Pérez MT, Sommaruga R (2011) Temporal changes in the dominance of major planktonic bacterial groups in an alpine lake: discrepancy with their contribution to bacterial production. Aquat Microb Ecol 63:161–170
Zwisler W, Selje N, Simon M (2003) Seasonal patterns of the bacterioplankton community composition in a large mesotrophic lake. Aquat Microb Ecol 31:211–225
Boucher D, Jardillier L, Debroas D (2006) Succession of bacterial community composition over two consecutive years in two aquatic systems: a natural lake and a lake-reservoir. FEMS Microbiol Ecol 55:79–97
Eiler A, Hayakawa DH, Rappe MS (2011) Non-random assembly of bacterioplankton communities in the subtropical North Pacific Ocean. Front Microbio 2:140, doi:10.3389/fmicb.2011.00140
Parveen B, Reveilliez JP, Mary I, Ravet V, Bronner G, J-Fo M, Domaizon I, Debroas D (2011) Diversity and dynamics of free-living and particle-associated Betaproteobacteria and Actinobacteria in relation to phytoplankton and zooplankton communities. FEMS Microbiol Ecol 77:461–476
Selje N, Simon M (2003) Composition and dynamics of particle-associated and free-living bacterial communities in the Weser estuary, Germany. Aquat Microb Ecol 30:221–237
Grossart H-P, Tang KW (2010) www.aquaticmicrobial.net. Commun Integr Biol 3:491–494
Rösel S, Grossart H-P (2012) Contrasting dynamics of activity and community composition of free-living and particle-associated bacteria in spring. Aquat Microb Ecol, doi:10.3354/ame01568
Hollibaugh JT, Wong PS, Murrell MC (2000) Similarity of particle-associated and free-living bacterial communities in northern San Francisco Bay, California. Aquat Microb Ecol 21:103–114
Beman JM, Sachdeva R, Fuhrman JA (2010) Population ecology of nitrifying Archaea and Bacteria in the Southern California Bight. Environ Microbiol 12:1282–1292
Bidle KD, Fletcher M (1995) Comparison of free-living and particle-associated bacterial communities in the Chesapeake Bay by stable low-molecular-weight RNA analysis. Appl Environ Microbiol 61:944–952
Rink B, Grüner N, Brinkhoff T, Ziegelmüller K, Simon M (2011) Regional patterns of bacterial community composition and biogeochemical properties in the southern North Sea. Aquat Microb Ecol 63:207–222
Muyzer G, Smalla K (1998) Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Van Leeuwenhoek 73:127–141
Riemann L, Winding A (2001) Community dynamics of free-living and particle-associated bacterial assemblages during a freshwater phytoplankton bloom. Microb Ecol 42:274–285
Simon M, Grossart H-P, Schweitzer B, Ploug H (2002) Microbial ecology of organic aggregates in aquatic ecosystems. Aquat Microb Ecol 28:175–211
Rösel S, Rychla A, Wurzbacher C, Grossart H-P (2012) Effects of pollen leaching and microbial degradation on organic carbon and nutrient availability in lake water. Aquat Sci 74:87–99
Jezbera J, Sharma AK, Brandt U, Doolittle WF, Hahn MW (2009) ‘Candidatus Planktophila limnetica’, an actinobacterium representing one of the most numerically important taxa in freshwater bacterioplankton. Int J Syst Evol Microbiol 59:2864–2869
Nelson CE (2008) Phenology of high-elevation pelagic bacteria: the roles of meteorologic variability, catchment inputs and thermal stratification in structuring communities. ISME J 3:13–30
Crump BC, Peterson BJ, Raymond PA, Amon RMW, Rinehart A, McClelland JW, Holmes RM (2009) Circumpolar synchrony in big river bacterioplankton. Proc Natl Acad Sci U S A 106:21208–21212
Stepanauskas R, Moran MA, Bergamaschi BA, Hollibaugh JT (2003) Covariance of bacterioplankton composition and environmental variables in a temperate delta system. Aquat Microb Ecol 31:85–98
Acknowledgments
We would like to thank Elke Mach and Johanna Dalchow as well as Michael Sachtleben and Roman Degebrodt for the technical assistance during sampling and for measurements of various limnetic parameters. Rainer Koschel and the Department ‘Limnology of Stratified Lakes’ of the IGB provided data on water chemistry. Lothar Krienitz and Peter Kasprzak are acknowledged for providing data on phytoplankton and zooplankton biomasses. Lake restoration and monitoring were financed by the Environmental Ministry of the German Federal State of Mecklenburg-Vorpommern and the city of Waren (Müritz). This study was financially supported by a grant of the German Research Foundation (DFG; GR 1540/12-1) and by the Leibniz Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
A) Concentrations of total phosphorus (TP) and soluble reactive phosphorus (SRP). Treatments for lake restoration are indicated by blue and grey bars. B) Primary production (PP, μg C L-1 d-1). C) Bacterial protein production (BPP, μg L-1 d-1) in Lake Tiefwaren from 2003 to 2009., BPP of total bacterial community (BPP total, black line) and of PA bacteria (BPP particle, blue line), standard deviation of replicates <15 % (not shown). (DOC 100 kb)
Figure S2
A) Total bacterial cell counts (× 106 mL-1). B) Bacterial abundances. percentages of Bacteria (EUB, red), Actinobacteria (HGC, blue) and acI-subcluster of Actinobacteria (acI, green) of total cell counts, error bars: standard deviation. (DOC 86 kb)
Figure S3
Maximum-likelihood phylogenetic tree of the obtained 16S rRNA gene sequences. (DOC 2645 kb)
Figure S4
Nonmetric multi-dimensional scaling (NMS) plot for environmental variables of Lake Tiefwaren. Data of physicochemical and biological parameters were normalised and resemblance was calculated using the Euclidean distance algorithm. Seasons are indicated by different colour-coded symbols according to seasonal clusters (spring, summer, autumn, winter). (DOC 395 kb)
Rights and permissions
About this article
Cite this article
Rösel, S., Allgaier, M. & Grossart, HP. Long-Term Characterization of Free-Living and Particle-Associated Bacterial Communities in Lake Tiefwaren Reveals Distinct Seasonal Patterns. Microb Ecol 64, 571–583 (2012). https://doi.org/10.1007/s00248-012-0049-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-012-0049-3