Abstract
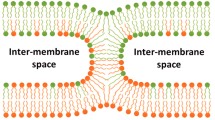
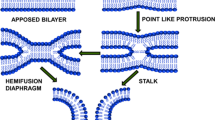
Thirty years ago, Klaus Arnold and others showed that the action of PEG in promoting cell–cell fusion was not due to such effects as surface absorption, cross-linking, solubilization, etc. Instead PEG acted simply by volume exclusion, resulting in an osmotic force driving membranes into close contact in a dehydrated region. This simple observation, based on a number of physical measurements and the use of PEG-based detergents that insert into membranes, spawned several important areas of research. One such area is the use of PEG to bring membranes into contact so that the role of different lipids and fusion proteins in membrane fusion can be examined in detail. We have summarized here insights into the fusion mechanism that have been obtained by this approach. This evidence indicates that fusion of model membranes (and probably cell membranes) occurs via severely bent lipidic structures formed at the point of sufficiently close contact between membranes of appropriate lipid composition. This line of research has also suggested that fusion proteins seem to catalyze fusion in part by reducing the free energy of hydrophobic interstices inherent to the lipidic fusion intermediate structures.




Similar content being viewed by others
References
Ahkong QF, Howell JI, Lucy JA, Safwat F, Davey MR, Cocking EC (1975) Fusion of hen erythrocytes with yeast protoplasts induced by polyethylene glycol. Nature 255:66–67
Armstrong RT, Kushnir AS, White JM (2000) The transmembrane domain of influenza hemagglutinin exhibits a stringent length requirement to support the hemifusion to fusion transition. J Cell Biol 151:425–437
Arnold K, Lvov Y, MSzogyi, Gyorgyi S (1980) Effects of poly(ethylene oxide) -containing surfactants on membrane-membrane interaction. Stud Biophys 113:7–14
Arnold K, Herrmann A, Gawrisch K, Pratsch L (1985) Mechanisms of poly(ethylene oxide)—induced fusion. Stud Biophys 110:135–141
Arnold K, Zschoernig O, Barthel D, Herold W (1990) Exclusion of poly(ethylene glycol) from liposome surfaces. Biochim Biophys Acta 1022:303–310
Bentz J, Ellens H (1988) Membrane fusion: kinetics and mechanisms. Colloids Surf 30:65–112
Boni LT, Hui SW (1987) The mechanism of P.E.G.—induced fusion in model membranes. In: Somers AE (ed) Cell fusion. Plenum, NY, pp 301–330
Boni LT, Stewart TP, Hui SW (1984) Alterations in phospholipid polymorphism by polyethylene glycol. J Membr Biol 80:91–104
Bowen ME, Weninger K, Brunger A, Chu S (2004) Single molecule observation of liposome–bilayer fusion thermally induced by SNAREs. Biophys J 87:3569–3584
Burgess SW, Massenburg D, Yates J, Lentz BR (1991) Poly(ethylene glycol)-induced lipid mixing but not fusion between synthetic phosphatidylcholine large unilamellar vesicles. Biochemistry 30:4193–4200
Burgess SW, McIntosh TJ, Lentz BR (1992) Modulation of poly(ethylene glycol)-induced fusion by membrane hydration: importance of interbilayer separation. Biochemistry 31:2653–2561
Chanturiya A, Chernomordik LV, Zimmerberg J (1997) Flickering fusion pores comparable with initial exocytotic pores occur in protein-free phospholipid bilayers. Proc Natl Acad Sci USA 94:14423–14428
Chen Z, Rand RP (1998) Comparative study of the effects of several n-alkanes on phospholipid hexagonal phases. Biophys J 74:944–952
Chen X, Arac D, Wang TM, Gilpin CJ, Zimmerberg J, Rizo J (2006) SNARE-mediated lipid mixing depends on the physical state of the vesicles. Biophys J 90:2062–2074
Chernomordik LV, Kozlov MM (2005) Membrane hemifusion: crossing a chasm in two leaps. Cell 123:375–282
Chernomordik LV, Zimmerberg J (1995) Bending membranes to the task: structural intermediates in bilayer fusion. Curr Opin Struct Biol 5:541–547
Chernomordik LV, Melikyan GB, Chizmadzhev YA (1987) Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim Biophys Acta 906:309–352
Chernomordik LV, Vogel SS, Sokoloff A, Onaran HO, Leikina EA, Zimmerberg J (1993) Lysolipids reversibly inhibit Ca(2+)−, GTP- and pH-dependent fusion of biological membranes. FEBS Lett 318:71–76
Chernomordik L, Chanturiya A, Green J, Zimmerberg J (1995) The hemifusion intermediate and its conversion to complete fusion: regulation by membrane composition. Biophys J 69:922–929
Clague MJ, Schoch C, Zech L, Blumenthal R (1990) Gating kinetics of pH-activated membrane fusion of vesicular stomatitis virus with cells: stopped-flow measurements by dequenching of octadecylrhodamine fluorescence. Biochemistry 29:1303–1308
Cleverley DZ, Lenard J (1998) The transmembrane domain in viral fusion: essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc Natl Acad Sci USA 95:3425–3230
Colotto A, Epand RM (1997) Structural study of the relationship between the rate of membrane fusion and the ability of the fusion peptide of influenza virus to perturb bilayers. Biochemistry 36:7644–7651
Dennison SM, Greenfield N, Lenard J, Lentz BR (2002) VSV transmembrane domain (TMD) peptide promotes PEG-mediated fusion of liposomes in a conformationally sensitive fashion. Biochemistry 41:14925–14934
Dennison SM, Bowen ME, Brunger AT, Lentz BR (2006) Neuronal SNAREs do not trigger fusion between synthetic membranes but do promote PEG-mediated membrane fusion. Biophys J 90:1661–1675
Ellens H, Bentz J, Szoka FC (1984) pH-induced destabilization of phosphatidylethanolamine-containing liposomes: role of bilayer contact. Biochemistry 23:1532–1538
Epand RM, Epand RF (1994) Relationship between the infectivity of influenza virus and the ability of its fusion peptide to perturb bilayers. Biochem Biophys Res Commun 202:1420–1425
Evans KO, Lentz BR (2002) Kinetics of lipid rearrangements during poly(ethylene glycol)-mediated fusion of highly curved unilamellar vesicles. Biochemistry 41:1241–1249
Evans E, Needham D (1988) Attraction between lipid bilayer membranes in concentrated solutions of non-absorbing polymers: comparison of membrane field theory. Macromolecules 21:1822–1831
Fix M, Melia TJ, Jaiswal JK, Rappoport JZ, You D, Sollner TH, Rothman JE, Simon SM (2004) Imaging single membrane fusion events mediated by SNARE proteins. Proc Natl Acad Sci USA 101:7311–7316
Giraudo CG, Hu C, You D, Slovic AM, Mosharov EV, Sulzer D, Melia TJ, Rothman JE (2005) SNAREs can promote complete fusion and hemifusion as alternative outcomes. J Cell Biol 170:249–260
Gruner SM, Tate MW, Kirk GL, So PT, Turner DC, Keane DT, Tilcock CP, Cullis PR (1988) X-ray diffraction study of the polymorphic behavior of N-methylated dioleoylphosphatidylethanolamine. Biochemistry 27:2853–2866
Han X, Jackson MB (2005) Electrostatic interactions between the syntaxin membrane anchor and neurotransmitter passing through the fusion pore. Biophys J 88:L20–L22
Han X, Wang CT, Bai J, Chapman ER, Jackson MB (2004) Transmembrane segments of syntaxin line the fusion pore of Ca2+-triggered exocytosis. Science 304:289–292
Haque ME, Lentz BR (2002) Influence of gp41 fusion peptide on the kinetics of poly(ethylene glycol)-mediated model membrane fusion. Biochemistry 41:10866–10876
Haque ME, Lentz BR (2004) Roles of curvature and hydrophobic interstice energy in fusion: studies of lipid perturbant effects. Biochemistry 43:3507–3517
Haque ME, McCoy AJ, Glenn J, Lee J, Lentz BR (2001a) Effects of hemagglutinin fusion peptide on poly(ethylene glycol)-mediated fusion of phosphatidylcholine vesicles. Biochemistry 40:14243–14251
Haque ME, McIntosh TJ, Lentz BR (2001b) Influence of lipid composition on physical properties and PEG-mediated fusion of curved and uncurved model membrane vesicles: “nature’s own” fusogenic lipid bilayer. Biochemistry 40:4340–4348
Haque ME, Koppaka V, Axelsen PH, Lentz BR (2005) Properties and structures of the influenza and HIV fusion peptides on lipid membranes: implications for a role in fusion. Biophys J 89:3183–3194
Hui SW, Stewart TP, Boni LT, Yeagle PL (1981) Membrane fusion through point defects in bilayers. Science 212:921–923
Jahn R, Lang T, Sudhof TC (2003) Membrane fusion. Cell 112:519–533
Kao KN, Michayluk MR (1974) A method for high-frequency intergeneric fusion of plant protoplasts. Planta 115:355–367
Kasbauer M, Lasic DD, Winterhalter M (1997) Polymer induced fusion and leakage of small unilamellar phospholipid vesicles: effect of surface grafted polyethylene-glycol in the presence of free PEG. Elsevier (CPL) 86:153–159
Katsov K, Muller M, Schick M (2004) Field theoretic study of bilayer membrane fusion. I. Hemifusion mechanism. Biophys J 87:3277–3290
Kozlovsky Y, Kozlov MM (2002) Stalk model of membrane fusion: solution of energy crisis. Biophys J 82:882–895
Kuhl T, Guo YQ, Alderfer JL, Berman AD, Leckband D, Israelachvili J, Hui SW (1996) Direct measurement of polyethylene glycol induced depletion attraction between lipid bilayers. Langmuir 12:3003–3014
Langosch D, Brosig B, Pipkorn R (2001) Peptide mimics of the vesicular stomatitis virus G-protein transmembrane segment drive membrane fusion in vitro. J Biol Chem 276:32016–32021
Lee J, Lentz BR (1997a) Evolution of lipidic structures during model membrane fusion and the relation of this process to cell membrane fusion. Biochemistry 36:6251–6259
Lee J, Lentz BR (1997b) Outer leaflet-packing defects promote poly(ethylene glycol)-mediated fusion of large unilamellar vesicles. Biochemistry 36:421–431
Lee J, Lentz BR (1998) Secretory and viral fusion may share mechanistic events with fusion between curved lipid bilayers. Proc Natl Acad Sci USA 95:9274–9279
Leikin SL, Kozlov MM, Chernomordik LV, Markin VS, Chizmadzhev YA (1987) Membrane fusion: overcoming of the hydration barrier and local restructuring. J Theor Biol 129:411–425
Lentz BR (1994) Polymer-induced membrane fusion: potential mechanism and relation to cell fusion events. Chem Phys Lipids 73:91–106
Lentz BR, Lee JK (1999) Poly(ethylene glycol) (PEG)-mediated fusion between pure lipid bilayers: a mechanism in common with viral fusion and secretory vesicle release? Mol Membr Biol 16:279–296
Lentz BR, McIntyre GF, Parks DJ, Yates JC, Massenburg D (1992) Bilayer curvature and certain amphipaths promote poly(ethylene glycol)- induced fusion of dipalmitoylphosphatidylcholine unilamellar vesicles. Biochemistry 31:2643–2653
Lentz BR, Talbot W, Lee J, Zheng LX (1997) Transbilayer lipid redistribution accompanies poly(ethylene glycol) treatment of model membranes but is not induced by fusion. Biochemistry 36:2076–2083
Lentz BR, Malinin V, Haque ME, Evans K (2000) Protein machines and lipid assemblies: current views of cell membrane fusion. Curr Opin Struct Biol 10:607–615
Lindau M, Almers W (1995) Structure and function of fusion pores in exocytosis and ectoplasmic membrane fusion. Curr Opin Cell Biol 7:509–517
MacDonald RI (1985) Membrane fusion due to dehydration by polyethylene glycol, dextran, or sucrose. Biochemistry 24:4058–4066
Malinin VS, Lentz BR (2002) Pyrene cholesterol reports the transient appearance of nonlamellar intermediate structures during fusion of model membranes. Biochemistry 41:5913–5919
Malinin VS, Lentz BR (2004) Energetics of vesicle fusion intermediates: comparison of calculations with observed effects of osmotic and curvature stresses. Biophys J 86:2951–2964
Malinin VS, Haque ME, Lentz BR (2001) The rate of lipid transfer during fusion depends on the structure of fluorescent lipid probes: a new chain-labeled lipid transfer probe pair. Biochemistry 40:8292–8299
Malinin VS, Frederik P, Lentz BR (2002) Osmotic and curvature stress affect PEG-induced fusion of lipid vesicles but not mixing of their lipids. Biophys J 82:2090–2100
Massenburg D, Lentz BR (1993) Poly(ethylene glycol)-induced fusion and rupture of dipalmitoylphosphatidylcholine large, unilamellar extruded vesicles. Biochemistry 32:9172–9280
McConnell H (1978) Dynamic properties of membranes; membrane immunochemistry. Adv Chemi Phys 39:249–285
McIntosh TJ, Simon SA (1994) Hydration and steric pressures between phospholipid bilayers. Annu Rev Biophys Biomol Struct 23:27–51
Melikyan GB, Jin H, Lamb RA, Cohen FS (1997) The role of the cytoplasmic tail region of influenza virus hemagglutinin in formation and growth of fusion pores. Virology 235:118–128
Melikyan GB, Lin S, Roth MG, Cohen FS (1999) Amino acid sequence requirements of the transmembrane and cytoplasmic domains of influenza virus hemagglutinin for viable membrane fusion. Mole Biol Cell 10:1821–1836
Nickel W, Weber T, McNew JA, Parlati F, Sollner TH, Rothman JE (1999) Content mixing and membrane integrity during membrane fusion driven by pairing of isolated v-SNAREs and t-SNAREs. Proc Natl Acad Sci USA 96:12571–12576
Oberhauser AF, Monck JR, Fernandez JM (1992) Events leading to the opening and closing of the exocytotic fusion pore have markedly different temperature dependencies. Kinetic analysis of single fusion events in patch-clamped mouse mast cells. Biophys J 61:800–809
Papahadjopoulos D, Hui S, Vail WJ, Poste G (1976a) Studies on membrane fusion. I. Interactions of pure phospholipid membranes and the effect of myristic acid, lysolecithin, proteins and dimethylsulfoxide. Biochim Biophys Acta 448:254–264
Papahadjopoulos D, Vail WJ, newton C, Nir S, Jacolson K, Poste G, Lato R (1976b) Studies on membrane fusion. III. The role of Ca-induced phase changes. BBA 465:579–598
Papahadjopoulos D, Vail WJ, Pangborn WA, Poste G (1976c) Studies on membrane fusion. II. Induction of fusion in pure phospholipid membranes by calcium ions and other divalent metals. Biochim Biophys Acta 448:265–283
Parente RA, Lentz BR (1986) Rate and extent of poly(ethylene glycol)-induced large vesicle fusion monitored by bilayer and internal contents mixing. Biochemistry 25:6678–6688
Poirier MA, Xiao W, Macosko JC, Chan C, Shin YK, Bennett MK (1998) The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat Struct Biol 5:765–769
Reese C, Heise F, Mayer A (2005) Trans-SNARE pairing can precede a hemifusion intermediate in intracellular membrane fusion. Nature 436:410–414
Saez R, Alonso A, Villena A, Goni FM (1982) Detergent-like properties of polyethyleneglycols in relation to model membranes. FEBS Lett 137:323–326
Schuette CG, Hatsuzawa K, Margittai M, Stein A, Riedel D, Kuster P, Konig M, Seidel C, Jahn R (2004) Determinants of liposome fusion mediated by synaptic SNARE proteins. Proc Natl Acad Sci USA 101:2858–2863
Siegel DP (1984) Inverted micellar structures in bilayer membranes. Formation rates and half-lives. Biophys J 45:399–420
Siegel DP (1993) Energetics of intermediates in membrane fusion: comparison of stalk and inverted micellar intermediate mechanisms. Biophys J 65:2124–2140
Siegel DP (1999) The modified stalk mechanism of Lamellar/Inverted phase transitions and its implications for membrane fusion. Biophys J 76:291–313
Siegel DP, Epand RM (1997) The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: implications for membrane fusion mechanisms. Biophys J 73:3089–3111
Siegel DP, Burns JL, Chestnut MH, Talmon Y (1989) Intermediates in membrane fusion and bilayer/nonbilayer phase transitions imaged by time-resolved cryo-transmission electron microscopy. Biophys J 56:161–169
Singer SJ, Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175:720–731
Spruce AE, Breckenridge LJ, Lee AK, Almers W (1990) Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron 4:643–654
Spruce AE, Iwata A, Almers W (1991) The first milliseconds of the pore formed by a fusogenic viral envelope protein during membrane fusion. Proc Natl Acad Sci USA 88:3623–3627
Struck DK, Hoekstra D, Pagano RE (1981) Use of resonance energy transfer to monitor membrane fusion. Biochemistry 20:4093–4099
Sutton RB, Fasshauer D, Jahn R, Brunger AT (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395:347–353
Suurkuusk J, Lentz BR, Barenholz Y, Biltonen RL, Thompson TE (1976) A calorimetric and fluorescent probe study of the gel-liquid crystalline phase transition in small, single-lamellar dipalmitoylphosphatidylcholine vesicles. Biochemistry 15:1393–1401
Szule JA, Jarvis SE, Hibbert JE, Spafford JD, Braun JEA, Zamponni GW, Wessel GM, Coorssen JR (2003) Calcium-triggered membrane fusion proceeds independently of specific presynaptic proteins. J Biol Chem 278:24251–24254
Talbot WA, Zheng LX, Lentz BR (1997) Acyl chain unsaturation and vesicle curvature alter outer leaflet packing and promote poly(ethylene glycol)-mediated membrane fusion. Biochemistry 36:5827–5836
Tse FW, Iwata A, Almers W (1993) Membrane flux through the pore formed by a fusogenic viral envelope protein during cell fusion. J Cell Biol 121:543–552
Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE (1998) Snarepins—minimal machinery for membrane fusion. Cell 92:759–772
Weinreb G, Lentz BR (2006) Kinetic model of PEG-mediated membrane fusion. Biophys J (in preparation)
Weninger K, Bowen ME, Chu S, Brunger AT (2003) Single-molecule studies of SNARE complex assembly reveal parallel and antiparallel configurations. Proc Natl Acad Sci USA 100:14800–14805
Wilschut J, Duzgunes N, Fraley R, Papahadjopoulos D (1980) Studies on the mechanism of membrane fusion: kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing of aqueous vesicle contents. Biochemistry 19:6011–6021
Wilschut J, Scholma J, Bental M, Hoekstra D, Nir S (1985) Ca2+-induced fusion of phosphatidylserine vesicles: mass action kinetic analysis of membrane lipid mixing and aqueous contents mixing. Biochim Biophys Acta 821:45–55
Wu JR, Lentz BR (1991) Mechanism of poly(ethylene glycol)-induced lipid transfer between phosphatidylcholine large unilamellar vesicles: a fluorescent probe study. Biochemistry 30:6780–6787
Wu H, Zheng L, Lentz BR (1996) A slight asymmetry in the transbilayer distribution of lysophosphatidylcholine alters the surface properties and poly(ethylene glycol)-mediated fusion of dipalmitoylphosphatidylcholine large unilamellar vesicles. Biochemistry 35:12602–12611
Xu Y, Zhang F, Su Z, McNew JA, Shin YK (2005) Hemifusion in SNARE-mediated membrane fusion. Nat Struct Mol Biol 12:417–422
Yang L, Huang HW (2003) A rhombohedral phase of lipid containing a membrane fusion intermediate structure. Biophys J 84:1808–1817
Zampighi GA, Zampighi LM, Fain N, Lanzavecchia S, Simon SA, Wright EM (2006) Conical electron tomography of a chemical synapse: vesicles docked to the active zone are hemi-fused. Biophys J (in press)
Zimmerberg J, Blumenthal R, Sarkar DP, Curran M, Morris SJ (1994) Restricted movement of lipid and aqueous dyes through pores formed by influenza hemagglutinin during cell fusion. J Cell Biol 127:1885–1894
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. K. Arnold on the occasion of his 65th birthday.
Rights and permissions
About this article
Cite this article
Lentz, B.R. PEG as a tool to gain insight into membrane fusion. Eur Biophys J 36, 315–326 (2007). https://doi.org/10.1007/s00249-006-0097-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-006-0097-z