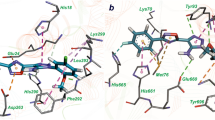
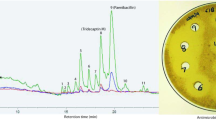
Abstract
The emergence of multidrug-resistant strains of pathogenic microorganisms and the slow progress in new antibiotic development has led in recent years to a resurgence of infectious diseases that threaten the well-being of humans. The result of many microorganisms becoming immune to major antibiotics means that fighting off infection by these pathogens is more difficult. The best strategy to get around drug resistance is to discover new drug targets, taking advantage of the abundant information that was recently obtained from genomic and proteomic research, and explore them for drug development. In this regard, aminoacyl-tRNA synthetases (ARSs) provide a promising platform to develop novel antibiotics that show no cross-resistance to other classical antibiotics. During the last few years there has been a comprehensive attempt to find the compounds that can specifically target ARSs and inhibit bacterial growth. In this review, the current status in the development of ARS inhibitors will be briefly summarized, based on their chemical structures and working mechanisms.




Similar content being viewed by others
References
Antonio M, McFerran N, Pallen MJ (2002) Mutations affecting the Rossman fold of isoleucyl-tRNA synthetase are correlated with low-level mupirocin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 46:438-442
Arnez JG, Moras D (1997) Structural and functional considerations of the aminoacylation reaction. Trends Biochem Sci 22:211–216
Baines PJ, Mellows G, Swaisland AJ, Tasker TC (1984) Mupirocin: its chemistry and metabolism. In: Mupirocin a novel topical antibiotic. Royal Society of Medicine, London, pp 13–22
Beecham Group PLC (1989) Antibacterial 1-normon-2-yl-heterocyclic compounds. US patent 4,861,788
Beecham Group PLC (1991) Antibacterial monic acid derivatives. US patent 5,041,567
Berge JM, Copley RC, Eggleston DS, Hamprecht DW, Jarvest RL, Mensah LM, O'Hanlon PJ, Pope AJ (2000) Inhibitors of bacterial tyrosyl tRNA synthetase: synthesis of four stereoisomeric analogues of the natural product SB-219383. Bioorg Med Chem Lett 10:1811–1814
Broom NJ, Cassels R, Cheng HY, Elder JS, Hannan PC, Masson N, O'Hanlon PJ, Pope A, Wilson JM (1996) The chemistry of pseudomonic acid. 17. Dual-action C-1 oxazole derivatives of pseudomonic acid having an extended spectrum of antibacterial activity. J Med Chem 39:3598–3600
Brown P, Eggleston DS, Haltiwanger RC, Jarvest RL, Mensah L, O'Hanlon PJ, Pope AJ (2001) Synthetic analogues of SB-219383. Novel C-glycosyl peptides as inhibitors of tyrosyl tRNA synthetase. Bioorg Med Chem Lett 11:711–714
Burbaum JJ, Schimmel P (1991) Structural relationships and the classification of aminoacyl-tRNA synthetases. J Biol Chem 266:16965–16968
Carcanague DR (1997) Novel derivatives of pseudomonic acid. Bioorg Med Chem Lett 7:2805–2808
Class YJ, Deshong P (1995) The pseudomonic acid. Chem Rev 95:1483–1857
Cookson BD (1998) The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother 41:11–18
Cubist Pharmaceuticals Inc (1998a) Aminoacyl adenylate minics as novel antimicrobial and antiparasitic agents. US patent 5,726,195
Cubist Pharmaceuticals Inc (1998b) Aminoacyl sulfamides for the treatment of hyperproliferative disorders. WO 98/41215
Cubist Pharmaceuticals Inc (2000a) Condensed imidazolidinones as tRNA synthetase inhibitors. WO 00/18772
Cubist Pharmaceuticals Inc (2000b) Tetracyclic heterocycles as antimicrobial. WO 00/17206
Cusack S (1997) Aminoacyl-tRNA synthetases. Curr Opin Struct Biol 7:881–889
Desjardins M, Garneau S, Desgagnes J, Lacoste L, Yang F, Lapointe J, Chenevert R (1998) Glutamyl adenylate analogues are inhibitors of glutamyl-tRNA synthetase. Bioorg Chem 26:1–13
Eriani G, Delarue M, Poch O, Gangloff J, Moras D (1990) Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature 347:203–206
Farmer TH, Gilbart J, Elson SW (1992) Biochemical basis of mupirocin resistance in strains of Staphylococcus aureus. J Antimicrob Chemother 30:587-596
Finn J, Hill J, Ram S, Morytko M, Yu X, Gimi R, Silverman J, Stein R, Lim A, Mak E, Gallant P, Wendler P, Rose S, Stevens A, Keith D (2001) Novel antibacterial agents targeting methionyl-tRNA synthetase: a chemlnformatic approach to convert HTS data into quality medicinal chemistry leads. In: Proceedings of the 41st Annual Interscience Conference on Antimicrobial Agents and Chemotherapy Chicago, Ill.
Forrest AK, Jarvest RL, Mensah LM, O'Hanlon PJ, Pope AJ, Sheppard RJ (2000) Aminoalkyl adenylate and aminoacyl sulfamate intermediate analogues differing greatly in affinity for their cognate Staphylococcus aureus aminoacyl tRNA synthetases. Bioorg Med Chem Lett 10:1871–1874
Fraser TH, Rich A (1975) Amino acids are not all initially attached to the same position on transfer RNA molecules. Proc Natl Acad Sci USA 72:3044–3048
Frugier M, Florentz C, Schimmel P, Giege R (1993) Triple aminoacylation specificity of a chimerized transfer RNA. Biochemistry 32:14053–14061
Heacock D, Forsyth CJ, Shiba K, Musiter-Forsyth K (1996) Synthesis and aminoacyl-tRNA synthetase inhibitory of prolyl adenylate analogs. Bioorg Chem 24:273–289
Hill J, Finn J, Wang Z, Silverman J, Oliver N, Gallant P, Wender P, Keith D (2001) Synthesis and activity of spirocyclic tetrahydrofurans as inhibitors of phenylalanine tRNA synthetase. In: Proceedings of the 41st Annual Interscience Conference on Antimicrobial Agents and Chemotherapy Chicago, Ill.
Houge-Frydrych CS, Readshaw SA, Bell DJ (2000a) SB-219383, a novel tyrosyl tRNA synthetase inhibitor from a Micromonospora sp. II. Structure determination. J Antibiot (Tokyo) 53:351–356
Houge-Frydrych CS, Gilpin ML, Skett PW, Tyler JW (2000b) SB-203207 and SB-203208, two novel isoleucyl tRNA synthetase inhibitors from a Streptomyces sp. II. Structure determination. J Antibiot (Tokyo) 53:364–372
Hughes J, Mellows G (1980) Interaction of pseudomonic acid A with Escherichia coli B isoleucyl-tRNA synthetase. Biochem J 191:209–219
Ibba M, Morgan S, Curnow AW, Pridmore DR, Vothknecht UC, Gardner W, Lin W, Woese CR, Soll D (1997) A euryarchaeal lysyl-tRNA synthetase: resemblance to class I synthetases. Science 278:1119–1122
Jarvest RL, Berge JM, Houge-Frydrych CS, Janson C, Mensah LM, O'Hanlon PJ, Pope A, Saldanha A, Qiu X (1999) Interaction of tyrosyl aryl dipeptides with S. aureus tyrosyl tRNA synthetase: inhibition and crystal structure of a complex. Bioorg Med Chem Lett 9:2859–2862
Jarvest RL, Berge JM, Berry V, Boyd HF, Brown MJ, Elder JS, Forrest AK, Fosberry AP, Gentry DR, Hibbs MJ, Jaworski DD, O'Hanlon PJ, Pope AJ, Rittenhouse S, Sheppard RJ, Slater-Radosti C, Worby A (2002) Nanomolar inhibitors of Staphylococcus aureus methionyl tRNA synthetase with potent antibacterial activity against gram-positive pathogens. J Med Chem 45:1959–1962
Klein LL, Yeung CM, Kurath P, Mao JC, Fernandes PB, Lartey PA, Pernet AG (1989) Synthesis and activity of nonhydrolyzable pseudomonic acid analogues. J Med Chem 32:151–160
Konishi M, Nishio M, Saitoh K, Miyaki T, Oki T, Kawaguchi H (1989) Cispentacin, a new antifungal antibiotic. I. Production, isolation, physico-chemical properties and structure. J Antibiot (Tokyo) 42:1749–1755
Konrad I, Roschenthaler R (1977) Inhibition of phenylalanine tRNA synthetase from Bacillus subtilis by ochratoxin A. FEBS Lett 83:341–347
Leberman R, Hartlein M, Cusack S (1991) Escherichia coli seryl-tRNA synthetase: the structure of a class 2 aminoacyl-tRNA synthetase. Biochim Biophys Acta 1089:287–298
Lee J, Kang SU, Kang MK, Chun MW, Jo YJ, Kwak JH, Kim S (1999) Methionyl adenylate analogues as inhibitors of methionyl-tRNA synthetase. Bioorg Med Chem Lett 9:1365–1370
Lee J, Kang SU, Kim SY, Kim SE, Jo YJ, Kim S (2001) Vanilloid and isovanilloid analogues as inhibitors of methionyl-tRNA and isoleucyl-tRNA synthetases. Bioorg Med Chem Lett 11:965–968
Merck & Co Inc (2000a) Novel prolines as antimicrobial agents. WO 00/66119
Merck & Co Inc (2000b) Novel catechols as antimicrobial agents. WO 00/66120
Morton TM, Johnston JL, Patterson J, Archer GL. (1995) Characterization of a conjugative staphylococcal mupirocin resistance plasmid. Antimicrob Agents Chemother 39:1272–1280
Nass G, Poralla K, Zahner H (1969) Effect of the antibiotic Borrelidin on the regulation of threonine biosynthetic enzymes in E. coli. Biochem Biophys Res Commun 34:84–91
Ogilvie A, Wiebauer K, Kersten W (1975) Inhibition of leucyl-transfer ribonucleic acid synthetasymol. Biochem J 152:511–515
Parr I, Hill J, Finn J, Yang D, Silverman J, Oliver N, Gallant P, Mak E, Stein R, Lim A, (2001) Synthesis and biological activity of novel di-alkylated thiazolidinone phenylalanyl-tRNA synthetase inhibitors. In: Proceedings of the 41st Annual Interscience Conference on Antimicrobial Agents and Chemotherapy Chicago, Ill.
Ribas de Pouplana L, Schimmel P (2001) Two classes of tRNA synthetases suggested by sterically compatible dockings on tRNA acceptor stem. Cell 104:191–193
Ruff M, Krishnaswamy S, Boeglin M, Poterszman A, Mitschler A, Podjarny A, Rees B, Thierry JC, Moras D (1991) Class II aminoacyl transfer RNA synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp). Science 52:1682–1689
Schimmel P, Giege R, Moras D, Yokoyama S (1993) An operational RNA code for amino acids and possible relationship to genetic code. Proc Natl Acad Sci USA 90:8763–8768
Schmitz FJ, Lindenlauf E, Hofmann AC, Fluit AC, Verhoef J, Heinz HP, Jones ME (1998) The prevalence of low- and high-level mupirocin resistance in staphylococci from 19 European hospitals. J Antimicrob Chemother 42:489–495
Sissler M, Eriani G, Martin F, Giege R, Florentz C (1997) Mirror image alternative interaction patterns of the same tRNA with either class I arginyl-tRNA synthetase or class II aspartyl-tRNA synthetase. Nucleic Acids Res 25:4899–4906
SmithKline Beecham PLC (1996a) (Hetero)-aryl ketones derivatives with antibacterial properties. US patent 5,536,745
SmithKline Beecham PLC (1996b) (Hetero)-aryl ketones derivatives with antibacterial properties. JP patent 9,157,269
SmithKline Beecham PLC (1997a) Mupirocinsulfamates with antibacterial activity. WO 97/05126
SmithKline Beecham PLC (1997b) Compounds with a sulfamoyl group and pharmaceutical compositions containing them. WO 97/35859
SmithKline Beecham PLC (1998a) Sulfamate derivatives with t-RNA synthetase inhibiting activity. WO 98/32765
SmithKline Beecham PLC (1998b) Enzymatic preparation of monic acids. US patent 5,726,049
SmithKline Beecham PLC (1999) Quinolones used as MRS inhibitors and bactericides. WO 99/55677
SmithKline Beecham PLC (2000a) 2-NH-Pyridones and pyrimidones as MRS inhibitors. WO 00/71524 A.
SmithKline Beecham PLC (2000b) Benzimidazole derivatives and their use as methionyl-tRNA synthetase inhibitors. WO 00/71522 A1
SmithKline Beecham PLC (2000c) Quinolones as t-RNA synthetase inhibitors and antibacterial agents. WO 00/21949
Stefanska AL, Fulston M, Houge-Frydrych CS, Jones JJ, Warr SR (2000a) A potent seryl tRNA synthetase inhibitor SB-217452 isolated from a Streptomyces species. J Antibiot (Tokyo) 53:1346–1353
Stefanska AL, Cassels R, Ready SJ, Warr SR (2000b) SB-203207 and SB-203208, two novel isoleucyl tRNA synthetase inhibitors from a Streptomyces sp. I. Fermentation, isolation and properties. J Antibiot (Tokyo) 53:357–363
Tanaka K, Tamaki M, Watanabe S (1969) Effect of furanomycin on the synthesis of isoleucyl-tRNA. Biochim Biophys Acta 195:244–245
Werner RG, Thorpe LF, Reuter W, Nierhaus KH (1976) Indolmycin inhibits prokaryotic tryptophanyl-tRNA ligase. Eur J Biochem 68:1–3
Yu XY, Hill JM, Yu G, Wang W, Kluge AF, Wendler P, Gallant P(1999) Synthesis and structure-activity relationships of a series of novel thiazoles as inhibitors of aminoacyl-tRNA synthetases. Bioorg Med Chem Lett 9:375–380
Yu XY, Hill JM, Yu G, Yang Y, Kluge AF, Keith D, Finn J, Gallant P, Silverman J, Lim A (2001) A series of quinoline analogues as potent inhibitors of C. albicans prolyl tRNA synthetase. Bioorg Med Chem Lett 11:541–544
Yun HJ, Lee SW, Yoon GM, Kim SY, Choi S, Lee YS, Choi EC, Kim S (2003) Prevalence and mechanisms of low- and high-level mupirocin resistance in staphylococci isolated from a Korean Hospital. J Antimicrob Chemother (in press)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S., Lee, S.W., Choi, EC. et al. Aminoacyl-tRNA synthetases and their inhibitors as a novel family of antibiotics. Appl Microbiol Biotechnol 61, 278–288 (2003). https://doi.org/10.1007/s00253-003-1243-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1243-5