Abstract
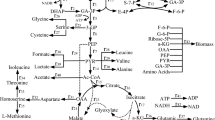
Metabolic flux analysis based on 13C-labeling experiments followed by the measurement of intracellular isotope distribution using both 2D NMR and GC-MS was carried out to investigate the effect of pyruvate kinase (pyk) gene knockout on the metabolism of Escherichia coli in continuous culture. In addition, the activities of 16 enzymes, and the concentrations of 5 intracellular metabolites, were measured as a function of time in batch culture as well as continuous culture. It was found that flux through phosphoenol pyruvate carboxylase and malic enzyme were up-regulated in the pykF − mutant as compared with the wild type, and acetate formation was significantly reduced in the mutant. In addition, flux through the phosphofructose kinase pathway was reduced and that through the oxidative pentose phosphate (PP) pathway increased in the mutant. This was evidenced by the corresponding enzyme activities, and the increase in the concentrations of phosphoenol pyruvate, glucose-6-phosphate and 6-phosphogluconate, etc. It was also found for continuous cultivation that the enzyme activities of the oxidative PP and Entner-Doudoroff pathways increased as the dilution rate increased for the pykF − mutant. To clarify the metabolism quantitatively, it was found to be quite important to integrate the information on intracellular metabolic flux distribution, enzyme activities and intracellular metabolite concentrations.







Similar content being viewed by others
References
Arauzo-Bravo MJ, Shimizu K (2003) An improved method for statistical analysis of metabolic flux analysis using isotopomer mapping matrices with analytical expressions. J Biotechnol (in press)
Buchholz A, Takors R, Wandrey C (2001) Quantification of intracellular metabolites in Escherichia coli K-12 using liquid chromatographic-electron spray ionization tandem mass spectrometric technique. Anal Biochem 295:129–137
Canonaco F, Hess TA, Heri S, Wang TT (2001) Metabolic flux response to phosphoglucose isomerse knock-out in Escherichia coli and impact of overall response of the soluble transhydrogenase UdhA. FEMS Microbiol Lett 204:247–252
Chambost JP, Fraenkel DG (1980) The use of 6-labeled glucose to assess futile cycling in Escherichia coli. J Biol Chem 255:2867–2869
Chao YP, Laio JC (1994) Metabolic responses to substrate futile cycling in Escherichia coli. J Biol Chem 269:5122–5126
Clark DP, Cronan JE (1996) Two-carbon compounds and fatty acids as carbon sources. In: Neidhardt FC et al (eds) Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd edn. ASM Press, Washington, D.C., pp 343–357
Coleman TF, Li Y, (1996) An interior, trust region approach for nonlinear minimization subject to bounds. SIAM J Optim 6:418–445
Cronan JE Jr, LaPorte D (1999) Tricarboxylic acid cycle and glyoxalate bypass. In: Neidhardt FC et al (eds) Escherichia coli and Salmonella typhimurium: molecular biology, 2nd edn. ASM press, Washington D.C., pp 343–357
Dalal F, Fraenkel DG (1983) Assessment of a futile cycle involving reconversion of fructose-6-phosphate to fructose-1,6-biphosphate during gluconeogenic growth of Escherichia coli. J Bacteriol 153:390–394
Datsenko KA, Wanner BL (2000) One step inactivation of chromosomal genes in Escherichia coli K-12 using PCR product. Proc Natl Acad Sci USA 97:6640–6645
Dauner M, Bailey J, Sauer U (2001) Metabolic flux analysis with a comprehensive isotopomer model in Bacillus subtilis. Biotechnol Bioeng 76:132–143
Emmerling M, Dauner M, Aaron P, Fiaux J, Hochuli M, Szyperski T, Wüthrich K, Bailey JE, Sauer U (2002) J Bacteriol 184:152–164
Fraenkel DG (1992) Genetics and intermediary metabolism. Annu Rev Genet 26:159–177
Fox DK, Presper KA, Adhya S, Roseman S, Ganges S (1992) Evidence of two promoters upstream of the pts operon: regulation by the cAMP receptor protein regulatory complex. Proc Natl Acad Sci USA 89:7056–7059
Gancedo JA, Gancedo C (1971) Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch Microbiol 76:132–138
Goldberg DE (1989) Genetic algorithms in search, optimization, and machine learning. Addison-Wesley, Reading, Mass.
KEGG (2002) Kyoto encyclopedia of genes and genomes. http://www.genome.ad.jp/kegg
Lamed R, Zeikus JG (1980) Glucose fermentation pathway of Thermonaerobium brockii. J Bacteriol 141:1252–1257
Lee PWN (1991) Mass isotopomer analysis: theoretical and practical considerations. Biol Mass Spectrom 20:451–458
Lehninger AL (1975) Biochemistry: the molecular basis of cell structure and function, 2nd edn. Worth, N.Y.
Marx A, De Graaf AA, Wiechert W, Eggeling L, Sahm H (1996) Determination of the fluxes in the central metabolism of Corynebacterium glutamicum by nuclear magnetic resonance spectroscopy combined with metabolic balancing. Biotechnol Bioeng 49:111–129
Möllney M, Wiechert W, Kowantzki D, de Graaf AA (1999) Bidirectional reaction steps in metabolic networks. IV Optimal design of isotopomer labeling experiments. Biotechnol Bioeng 66:86–103
Neidhardt FC, Umbarger HE (1999) Chemical composition of Escherichia coli. In: Neidhardt FC et al (eds) Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd edn. ASM Press, Washington D.C.
Oh MK, Rohlon L, Kao K, Liao JC (2002) Global expression profiling of acetate-grown E. coli. J Biol Chem 277:13175–13183
Park SJ, Cotter PA, Gunsalus RP (1995a) Regulation of malate dehydrogenase (mdh) gene expression in Escherichia coli in response to oxygen, carbon and heme availability. J Bacteriol 177:6652–6656
Parvin R (1969) Citrate synthase from rat liver. Methods Enzymol 13:16–19
Plumbridge J (1999) Expression of the phosphotransferase system both mediates and is mediated by Mlc regulation in Escherichia coli. Mol Microbiol 33:260–273
Ponce E, Flores N, Martinez A, Bolivar F, Valle F (1995) Cloning of the two pyruvate kinase isoenzyme structural genes from Escherichia coli: the relative role of these enzymes in pyruvate biosynthesis. J Bacteriol 177:5719–5722
Ponce E, Martinez A, Bolivar F, Valle F (1998) Stimulation of glucose catabolism through the pentose pathway by the absence of the two pyruvate kinase isoenzymes in Escherichia coli. Biotechnol Bioeng 58:292–295
Reuse HD, Danchin A (1988) The ptsH, ptsI, and crr genes of the Escherichia coli phosphoenol pyruvate-dependent phosphotransferase system: a complex operon with several modes of transcription. J Bacteriol 170:3827–3837
Saier MH, Ramseier TM (1996) The catabolic repressor/activator (cra) protein of enteric bacteria. J Bacteriol 178:341–347
Salas M, Vinuela E, Sols A (1965) Spontaneous and enzymatic anomerization of glucose-6-phosphate and anomeric specificity of related enzymes. J Biol Chem 240:561–568
Samuelov NS, Lamed R, Lowe S, Zeikus JG (1991) Influence of CO2-HCO3- levels and pH on growth, succinate production and enzyme activities of Anaerbiospirillum succiniciproducens. Appl Environ Microbiol 57:3013–3019
Sauer U, Lasko DR, Flaux JL, Hochuli M, Glaser R, Szyperski T, Wüthrich K, Bailey JE (1999) Metabolic flux ratio analysis of genetic and environmental modulations of Escherichia coli central carbon metabolism. J Bacteriol 181:6679–6688
Schaefer U, Boos W, Takors R, Weuster-Botz D (1999) Automated sampling device for monitoring intracellular metabolite dynamics. Anal Biochem 270:88–96
Schmidt K, Carlsen M, Nielsen J, Villadsen J (1997) Modeling isotopomer distributions in biochemical networks using isotoper mapping matrices. Biotechnol Bioeng 55:831–840
Schmidt K, Nielsen J, Villadsen J (1999) Quantitative analysis of metabolic fluxes in Escherichia coli using two-dimensional NMR spectroscopy and complete isotopomer models. J Biotechnol 71:175–190
Sridhar J, Eiteman M, Wiegel JW (2000) Elucidation of enzymes in fermentation pathways used by Clostridium thermosuccino genes growing on inulin. J Appl Environ Microbiol 66:246–251
Steiner P, Fussenegger M, Bailey JE, Sauer U (1998) Cloning expression of the Zymomonas mibilis pyruvate kinase gene in Escherichia coli. Gene 220:31–38
Szyperski T (1995) Biosynthetically directed fractional 13C-labeling of proteinogenic amino acid. Eur J Biochem 232:443–448
Szyperski T, Glaser RW, Hochuli M, Fiaux J, Sauer U, Bailey JE, Wüthrich K (1999) Bioreaction network topology and metabolic flux ratio analysis by biosynthetic fractional 13C labeling and two-dimensional NMR spectroscopy. Metabol Eng 1:189–197
Tao H, Bausch C, Richmond C, Blatner RF, Conway T, (1999) Functional genomics: expression analysis of Escherichia coli growing on minimal and rich media. J Bacteriol 181:6425–6440
Van der Werf MJ, Guettle MV, Jain MK, Zeikus JG (1997) Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z. Arch Microbiol 147:195–200
Van Winden W, Schipper D, Verheijen P, Heijnen J (2001) Innovations in generation and analysis of 2D [13C, 1H] COSY NMR spectra for metabolic flux analysis purposes. Metabol Eng 3:322–343
Walsh K, Koshland DE (1985) Branch point control by the phosphorylation state of isocitrate dehydrogenase. J Biol Chem 260:8430–8437
Wiechert W, de Graaf AA (1997) Bidirectional reaction steps in metabolic networks: I. Modeling and simulation of carbon isotope labeling experiments. Biotechnol Bioeng 55:101–117
Wiechert W, Siefke C, de Graff AA, Marx A (1997) Bidirectional reaction steps in metabolic networks: II. Flux estimation and statistical analysis. Biotechnol Bioeng 55:118–135
Yang C, Hua Q, Shimizu K (2003) Analysis of E. coli anaplerotic metabolism and its regulation mechanisms from the metabolic responses to altered dilution rates and ppc knockout. Meas Biotechnol Bioeng (in press)
Zhu T, Phalakornkule C, Koepsel RR, Domach MM, Ataai MM (2001) Cell growth and by product formation in pyruvate kinase mutant of E. coli. Biotechnol Prog 17:624–628
Acknowledgement
It is acknowledged that this research was supported in part by a grant from New Energy and Industrial Technology Development Organization of Japan (NEDO) of the Ministry of Economy, Trade and Industry of Japan (Development of a Technological Infrastructure for Industrial Bioprocess Project).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al Zaid Siddiquee, K., Arauzo-Bravo, M.J. & Shimizu, K. Metabolic flux analysis of pykF gene knockout Escherichia coli based on 13C-labeling experiments together with measurements of enzyme activities and intracellular metabolite concentrations. Appl Microbiol Biotechnol 63, 407–417 (2004). https://doi.org/10.1007/s00253-003-1357-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1357-9