Abstract
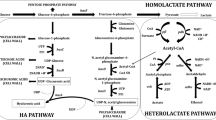
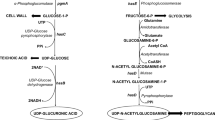
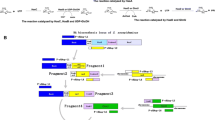
Hyaluronic acid (HA) production was metabolically engineered in Lactococcus lactis by introducing the HA synthetic machinery from the has operon of the pathogenic bacterium Streptococcus zooepidemicus. This study shows that the insertion of uridine diphosphate (UDP)-glucose pyrophosphorylase (hasC) gene in addition to the HA synthase (hasA) and UDP-glucose dehydrogenase (hasB) genes has a significant impact on increasing HA production. The recombinant L. lactis NZ9000 strain transformed with the plasmid pSJR2 (co-expressing hasA and hasB genes only) produced a maximum of 107 mg/l HA in static flask experiments with varying initial glucose concentrations, while the corresponding experiments with the transformant SJR3 (co-expressing hasA, hasB, and hasC genes) gave a maximum yield of 234 mg/l HA. The plasmid cloned with the insertion of the full has operon comprising of five different genes (hasA, hasB, hasC, hasD, and hasE) exhibited structural instability. The HA yield was further enhanced in batch bioreactor experiments with controlled pH and aeration, and a maximum of 1.8 g/l HA was produced by the SJR3 culture.







Similar content being viewed by others
References
Ashbaugh CD, Sebastián A, Wessels MR (1998) Molecular analysis of the capsule gene region of group A Streptococcus: the hasAB genes are sufficient for capsule expression. J Bacteriol 180:4955–4959
Balazs EA, Denlinger JL (1989) Clinical uses of hyaluronan. Ciba Found Symp 143:265–275
Bitter T, Muir HM (1962) A modified uronic acid carbazole reaction. Anal Biochem 4:330–334
Chen WY, Marcellin E, Hung J, Nielsen LK (2009) Hyaluronan molecular weight is controlled by UDP-N-acetylglucosamine concentration in Streptococcus zooepidemicus. J Biol Chem 284:18007–18014
Chien LJ, Lee CK (2007) Hyaluronic acid production by recombinant Lactococcus lactis. Appl Microbiol Biotechnol 77:339–349
Chong BF, Blank LM, McLaughin R, Nielsen LK (2005) Microbial hyaluronic acid production. Appl Microbiol Biotechnol 66:341–351
DeAngelis PL, Jing W, Drake RR, Achyuthan AM (1998) Identification and molecular cloning of a unique hyaluronan synthase from Pasteurella multocida. J Biol Chem 273:8454–8458
Gao HJ, Du GC, Chen J (2006) Analysis of metabolic fluxes for hyaluronic acid (HA) production by Streptococcus zooepidemicus. World J Microbiol Biotechnol 22:399–408
Gilli R, Kacurakova M, Mathlouthi M, Navarini L, Paoletti S (1994) FTIR studies of sodium hyaluronate and its oligomers in the amorphous solid phase and in aqueous solution. Carbohydr Res 263:315–326
Holo H, Nes IF (1989) High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol 55:3119–3123
Johns MR, Goh L, Oeggerli A (1994) Effect of pH, agitation and aeration on hyaluronic acid production by Streptococcus zooepidemicus. Biotech Lett 16:507–512
Kim JH, Yoo SJ, Oh DK, Kweon YG, Park DW, Lee CH, Gil GH (1996) Selection of a Streptococcus equi mutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid. Enzym Microb Technol 19:440–445
Kleerebezem M, Kranenburg RV, Tuinier R, Boels IC, Zoon P, Looijesteijn E, Hugenholtz J, Vos WMD (1999) Exopolysaccharide production by L. lactis from genetic engineering to improved rheology? Antonie Von Leeuwenhoek 76:357–365
Kogan G, Šoltés L, Stern R, Gemeiner P (2006) Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett 29:1–17
Kok J, Buist G, Zomer AL, Van Hijum AAFT, Kuipers OP (2005) Comparative and functional genomics of lactococci. FEMS Microbiol Rev 29:411–433
Kuipers OP, de Ruyter P, Kleerebezem M, de Vos WM (1998) Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol 64:15–21
Magee C, Nurminskaya M, Linsenmayer TF (2001) UDP-glucose pyrophosphorylase: up-regulation in hypertrophic cartilage and role in hyaluronan synthesis. Biochem J 360:667–674
Mao Z, Chen R (2007) Recombinant synthesis of hyaluronan by Agrobacterium sp. Biotechnol Prog 23:1038–1042
Mao Z, Shin H-D, Chen R (2009) A recombinant E. coli bioprocess for hyaluronan synthesis. Appl Microbiol Biotechnol 84:63–69
Mierau I, Kleerebezem M (2005) 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol 68:705–717
Pongtharangkul ET, Demirci A (2008) Evaluation of culture medium for nisin production in a repeated-batch biofilm reactor. Biotechnol Prog 22:217–224
Rangaswamy V, Jain D (2008) An efficient process for production and purification of hyaluronic acid from Streptococcus equi subsp. zooepidemicus. Biotechnol Lett 30:493–496
Ribeiro LA, Azevedo V, Le Loir Y, Oliveira SC, Dieye Y, Piard JC, Gruss A, Langella P (2002) Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: a first step towards food-grade live vaccines against brucellosis. Appl Environ Microbiol 68:910–916
Ryan MP, Rea MC, Hill C, Ross RP (1996) An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin. Appl Environ Microbiol 62:612–619
Sheng JZ, Ling PX, Zhu XQ, Guo XP, Zhang TM, He YL, Wang FS (2009) Use of induction promoters to regulate hyaluronan synthesis and UDP-glucose-6-dehydrogenase of Streptococcus zooepidemicus expression in Lactococcus lactis: a case study of the regulation mechanism of hyaluronic acid polymer. J Appl Microbiol 107:136–144
Sriraman K, Jayaraman G (2006) Enhancement of recombinant streptokinase production in Lactococcus lactis by suppression of acid tolerance response. Appl Microbiol Biotechnol 72:1202–1209
Wegmann U, O'Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, Ventura M, Goesmann A, Gasson MJ, Kuipers OP, van Sinderen D, Kok J (2007) Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol 189:3256–3270
Weigel PH, DeAngelis PL (2007) Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem 282:36777–36781
Widner B, Behr R, Dollen SV, Tang M, Heu T, Sloma A, Sternberg D, DeAngelis PL, Weigel PH, Brown S (2005) Hyaluronic acid production in Bacillus subtilis. Appl Environ Microbiol 77:3747–3752
Yu H, Stephanopoulos G (2008) Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metab Eng 10:24–32
Zhoua XX, Lib WF, Mab GX, Pana YJ (2006) The nisin-controlled gene expression system: construction, application and improvements. Biotechnol Adv 24:285–295
Acknowledgments
The authors would like to acknowledge the financial support provided by Indian Institute of Technology Madras for this project and Dr. Kalpana Sriraman for her valuable comments and suggestions toward this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary data, Annexure 1
(DOC 25 kb)
Rights and permissions
About this article
Cite this article
Prasad, S.B., Jayaraman, G. & Ramachandran, K.B. Hyaluronic acid production is enhanced by the additional co-expression of UDP-glucose pyrophosphorylase in Lactococcus lactis . Appl Microbiol Biotechnol 86, 273–283 (2010). https://doi.org/10.1007/s00253-009-2293-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2293-0