Abstract
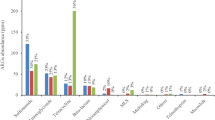
As antibiotic resistance continues to spread globally, there is growing interest in the potential to limit the spread of antibiotic resistance genes (ARGs) from wastewater sources. In particular, operational conditions during sludge digestion may serve to discourage selection of resistant bacteria, reduce horizontal transfer of ARGs, and aid in hydrolysis of DNA. This study applied metagenomic analysis to examine the removal efficiency of ARGs through thermophilic and mesophilic anaerobic digestion using bench-scale reactors. Although the relative abundance of various ARGs shifted from influent to effluent sludge, there was no measureable change in the abundance of total ARGs or their diversity in either the thermophilic or mesophilic treatment. Among the 35 major ARG subtypes detected in feed sludge, substantial reductions (removal efficiency >90 %) of 8 and 13 ARGs were achieved by thermophilic and mesophilic digestion, respectively. However, resistance genes of aadA, macB, and sul1 were enriched during the thermophilic anaerobic digestion, while resistance genes of erythromycin esterase type I, sul1, and tetM were enriched during the mesophilic anaerobic digestion. Efflux pump remained to be the major antibiotic resistance mechanism in sludge samples, but the portion of ARGs encoding resistance via target modification increased in the anaerobically digested sludge relative to the feed. Metagenomic analysis provided insight into the potential for anaerobic digestion to mitigate a broad array of ARGs.



Similar content being viewed by others
References
Ashbolt NJ, Amezquita A, Backhaus T, Borriello P, Brandt KK, Collignon P, Coors A, Finley R, Gaze WH, Heberer T, Lawrence JR, Larsson DG, McEwen SA, Ryan JJ, Schonfeld J, Silley P, Snape JR, Van den Eede C, Topp E (2013) Human health risk assessment (HHRA) for environmental development and transfer of antibiotic resistance. Environ Health Perspect 121:993–1001
Baquero F, Martínez JL, Cantón R (2008) Antibiotics and antibiotic resistance in water environments. Curr Opin Biotechnol 19:260–265
Chen B, Yang Y, Liang X, Yu K, Zhang T, Li X (2013) Metagenomic profiles of antibiotic resistance genes (ARGs) between human impacted estuary and deep ocean sediments. Environ Sci Technol 47:12753–12760
Diehl DL, LaPara TM (2010) Effect of temperature on the fate of genes encoding tetracycline resistance and the integrase of class 1 integrons within anaerobic and aerobic digesters treating municipal wastewater solids. Environ Sci Technol 44:9128–9133
Eaton AD, Franson MAH (2005) Standard methods for the examination of water and wastewater. American Public Health Association. Washington, D.C. USA
Ghosh S, Ramsden SJ, LaPara TM (2009) The role of anaerobic digestion in controlling the release of tetracycline resistance genes and class 1 integrons from municipal wastewater treatment plants. Appl Microbiol Biotechnol 84:791–796
Koczura R, Mokracka J, Jabłońska L, Gozdecka E, Kubek M, Kaznowski A (2012) Antimicrobial resistance of integron-harboring Escherichia coli isolates from clinical samples, wastewater treatment plant and river water. Sci Total Environ 414:680–685
Kristiansson E, Fick J, Janzon A, Grabic R, Rutgersson C, Weijdegård B, Söderström H, Larsson DGJ (2011) Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PLoS ONE 6:e17038
Kümmerer K (2009a) Antibiotics in the aquatic environment – a review – part I. Chemosphere 75:417–434
Kümmerer K (2009b) Antibiotics in the aquatic environment – a review – part II. Chemosphere 75:435–441
Kwietniewska E, Tys J (2014) Process characteristics, inhibition factors and methane yields of anaerobic digestion process, with particular focus on microalgal biomass fermentation. Renew Sust Energ Rev 34:491–500
LaPara TM, Burch TR, McNamara PJ, Tan DT, Yan M, Eichmiller JJ (2011) Tertiary-treated municipal wastewater is a significant point source of antibiotic resistance genes into Duluth-Superior Harbor. Environ Sci Technol 45:9543–9549
Levy S (1982) Microbial resistance to antibiotics. An evolving and persistent problem. Lancet Infect Dis 320:83–88
Ma Y, Wilson CA, Novak JT, Riffat R, Aynur S, Murthy S, Pruden A (2011) Effect of various sludge digestion conditions on sulfonamide, macrolide, and tetracycline resistance genes and class I integrons. Environ Sci Technol 45:7855–7861
Martinez JL (2008) Antibiotics and antibiotic resistance genes in natural environments. Science 321:365–367
Mulvey MR, Simor AE (2009) Antimicrobial resistance in hospitals: how concerned should we be? CMAJ 180:408–415
Munir M, Wong K, Xagoraraki I (2011) Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res 45:681–693
Novak JT, Chon DH, Curtis BA, Doyle M (2007) Biological solids reduction using the cannibal process. Water Environ Res 79:2380–2386
Pruden A, Pei R, Storteboom H, Carlson KH (2006) Antibiotic resistance genes as emerging contaminants: studies in Northern Colorado. Environ Sci Technol 40:7445–7450
Pruden A, Larsson DGJ, Amézquita A, Collignon P, Brandt KK, Graham DW, Lazorchak JM, Suzuki S, Silley P, Snape JR, Topp E, Zhang T, Zhu YG (2013) Management options for reducing the release of antibiotics and antibiotic resistance genes to the environment. Environ Health Perspect 121:878–885
Riesenfeld CS, Goodman RM, Handelsman J (2004) Uncultured soil bacteria are a reservoir of new antibiotic resistance genes. Environ Microbiol 6:981–989
Rysz M, Mansfield WR, Fortner JD, Alvarez PJ (2013) Tetracycline resistance gene maintenance under varying bacterial growth rate, substrate and oxygen availability, and tetracycline concentration. Environ Sci Technol 47:6995–7001
Sahlstrom L (2003) A review of survival of pathogenic bacteria in organic waste used in biogas plants. Bioresour Technol 87:161–166
Storteboom H, Arabi M, Davis JG, Crimi B, Pruden A (2010) Tracking antibiotic resistance genes in the south Platte river basin using molecular signatures of urban, agricultural, and pristine sources. Environ Sci Technol 44:7397–7404
Summers AO (2006) Genetic linkage and horizontal gene transfer, the roots of the antibiotic multi-resistance problem. Anim Biotechnol 17:125–135
Webber MA (2002) The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 51:9–11
Wright GD (2007) The antibiotic resistome: the nexus of chemical and genetic diversity. Nat Rev Microbiol 5:175–186
Wright GD (2010) Antibiotic resistance in the environment: a link to the clinic? Curr Opin Microbiol 13:589–594
Xu J, Xu Y, Wang H, Guo C, Qiu H, He Y, Zhang Y, Li X, Meng W (2014) Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere. doi:10.1016/j.chemosphere.2014.02.040
Yang Y, Zhang T, Zhang XX, Liang DW, Zhang M, Gao DW, Zhu HG, Huang QG, Fang HHP (2011) Quantification and characterization of β-lactam resistance genes in 15 sewage treatment plants from East Asia and North America. Appl Microbiol Biotechnol 95:1351–1358
Yang Y, Li B, Ju F, Zhang T (2013) Exploring variation of antibiotic resistance genes in activated sludge over a four-year period through a metagenomic approach. Environ Sci Technol 47:10197–10205
Yang Y, Li B, Zou S, Fang HHP, Zhang T (2014a) Fate of antibiotic resistance genes in sewage treatment plant revealed by metagenomic approach. Water Res 62:97–106
Yang Y, Yu K, Xia Y, Lau FT, Tang DT, Fung WC, Fang HHP, Zhang T (2014b) Metagenomic analysis of sludge from full-scale anaerobic digesters operated in municipal wastewater treatment plants. Appl Microbiol Biotechnol 98:5709–5718
Zhang T, Zhang M, Zhang X, Fang HHP (2009a) Tetracycline resistance genes and tetracycline resistant lactose-fermenting Enterobacteriaceae in activated sludge of sewage treatment plants. Environ Sci Technol 43:3455–3460
Zhang XX, Zhang T, Fang HHP (2009b) Antibiotic resistance genes in water environment. Appl Microbiol Biotechnol 82:397–414
Zhang T, Zhang XX, Ye L (2011) Plasmid metagenome reveals high levels of antibiotic resistance genes and mobile genetic elements in activated sludge. PLoS ONE 6:e26041
Acknowledgments
This study was financially supported by the Research Grants Council of Hong Kong (HKU7201/11E). Ying Yang thanks the University of Hong Kong for the postgraduate studentship.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 309 kb)
Rights and permissions
About this article
Cite this article
Zhang, T., Yang, Y. & Pruden, A. Effect of temperature on removal of antibiotic resistance genes by anaerobic digestion of activated sludge revealed by metagenomic approach. Appl Microbiol Biotechnol 99, 7771–7779 (2015). https://doi.org/10.1007/s00253-015-6688-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6688-9