Abstract
Purpose
To examine the usefulness of dual-echo dual-flip angle spoiled gradient recalled (SPGR) magnetic resonance imaging (MRI) technique in quantifying muscle fat fraction (MFF) of pelvic and thighs muscles as a marker of disease severity in boys with Duchenne muscular dystrophy (DMD), by correlating MFF calculation with clinical assessments. We also tried to identify characteristic patterns of disease distribution.
Materials and methods
Twenty consecutive boys (mean age, 8.6 years ± 2.3 [standard deviation, SD]; age range, 5–15 years; median age, 9 years;) with DMD were evaluated using a dual-echo dual-flip angle SPGR MRI technique, calculating muscle fat fraction (MFF) of eight muscles in the pelvic girdle and thigh (gluteus maximus, adductor magnus, rectus femoris, vastus lateralis, vastus medialis, biceps femoris, semitendinosus, and gracilis). Color-coded parametric maps of MFF were also obtained. A neurologist who was blinded to the MRI findings performed the clinical assessments (patient age, Medical Research Council score, timed Gower score, time to run 10 m). The relationships between mean MFF and clinical assessments were investigated using Spearman’s rho coefficient. Positive and negative correlations were evaluated and considered significant if the P value was < 0.05.
Results
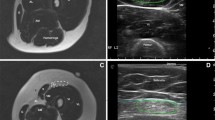
The highest mean MFF was found in the gluteus maximus (mean, 46.3 % ± 24.5 SD), whereas the lowest was found in the gracilis muscle (mean, 2.7 % ± 4.7 SD). Mean MFF of the gluteus maximus was significantly higher than that of the other muscles (P < 0.01), except for the adductor magnus and biceps muscles. A significant positive correlation was found between the mean MFF of all muscles and the patients age (20 patients; P < 0.005), Medical Research Council score (19 patients; P < 0.001), timed Gower score (17 patients; P < 0.03), and time to run 10 m (20 patients; P < 0.001). A positive correlation was also found between the mean MFF of the gluteus maximus muscle and the timed Gower score. Color-coded maps provided an efficient visual assessment of muscle fat content and its heterogeneous distribution.
Conclusion
Muscle fat fraction calculation and mapping using the dual-echo dual-flip angle SPGR MRI technique are useful markers of disease severity and permit patterns of disease distribution to be identified in patients with DMD.






Similar content being viewed by others
References
Dubowitz V. The muscular dystrophies. In: Muscle disorders in childhood. 2nd ed. London: Saunders, 1995; 39–42.
Mercuri E, Mayhew A, Muntoni F, et al. On behalf of the TREAT-NMD Neuromuscular Network. Towards harmonisation of outcome measures for DMD and SMA within TREAT-NMD; report of three expert workshops: TREATNMD/ENMC workshop on outcome measures, 12th–13th May 2007, Naarden, The Netherlands; TREAT-NMD workshop on outcome measures in experimental trials for DMD, 30th June–1st July 2007, Naarden, The Netherlands; Conjoint Institute of Myology TREAT-NMD Meeting on physical activity monitoring in neuromuscular disorders, 11th July 2007, Paris, France. Neuromuscul Disord. 2008;18:894–903.
Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. The design of the protocol. Muscle Nerve. 1981;4:186–97.
Scott OM, Hyde SA, Goddard C, Dubowitz V. Quantitation of muscle function in children: a prospective study in Duchenne muscular dystrophy. Muscle Nerve. 1982;5:291–301.
Florence JM, Pandya S, King WM, et al. Intrarater reliability of manual muscle test (Medical Research Council scale) grades in Duchenne’s muscular dystrophy. Phys Ther. 1992;72:115–22.
Mayhew JE, Florence JM, Mayhew TP, et al. Reliable surrogate outcome measures in multicenter clinical trials of Duchenne muscular dystrophy. Muscle Nerve. 2007;35:36–42.
Mazzone ES, Messina S, Vasco G, et al. Reliability of the North Star Ambulatory Assessment in a multicentric setting. Neuromuscul Disord. 2009;19:458–61.
Mazzone E, Martinelli D, Berardinelli A, et al. North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2010;20:712–6.
Schreiber A, Smith WL, Ionasescu V, et al. Magnetic resonance imaging of children with Duchenne muscular dystrophy. Pediatr Radiol. 1987;17:495–7.
Liu GC, Jong YJ, Chiang CH, Jaw TS. Duchenne muscular dystrophy: MR grading system with functional correlation. Radiology. 1993;186:475–80.
Marden FA, Connolly AM, Siegel MJ, Rubin DA. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol. 2005;34:140–8.
Huang Y, Majumdar S, Genant HK, et al. Quantitative MR relaxometry study of muscle composition and function in Duchenne muscular dystrophy. J Magn Reson Imaging. 1994;4:59–64.
Phoenix J, Betal D, Roberts N, Helliwell TR, Edwards RH. Objective quantification of muscle and fat in human dystrophic muscle by magnetic resonance image analysis. Muscle Nerve. 1996;19:302–10.
Kim HK, Laor T, Horn PS, Racadio JM, Wong B, Dardzinski BJ. T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments. Radiology. 2010;225:899–908.
Wren TA, Bluml S, Tseng-Ong L, Gilsanz V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR Am J Roentgenol. 2008;190:W8–12.
Gaeta M, Scribano E, Mileto A, et al. Muscle fat fraction in neuromuscular disorder: dual-echo dual-flip-angle spoiled gradient-recalled MR imaging technique for quantification—a feasibility study. Radiology. 2011;259:487–94.
Gaeta M, Mileto A, Mazzeo A, et al. MRI findings, patterns of disease distribution, and muscle fat fraction calculation in five patients with Charcot-Marie-Tooth type 2 F disease. Skeletal Radiol 2011;doi:10.1007/s00256-011-1199-y.
Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–16.
Muntoni F, Bushby KD, Van Ommen G. 149th ENMC International Workshop and 1st Treat-NMD Workshop on “Planning phase I/II clinical trials using systematically delivered antisense oligonucleotides in Duchenne muscular dystrophy”. Neuromuscul Disord. 2008;18:268–75.
Eagle M, Baudouin S, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12:926–9.
Wagner KR. Approaching a new age in Duchenne muscular dystrophy treatment. Neurotherapeutics. 2008;5:583–9.
Yokota T, Takeda S, Lu QL, Partridge TA, Nakamura A, Hoffman EP. A renaissance of antisense oligonucleotide drugs in neurology: exon skipping breaks new ground. Arch Neurol. 2009;66:32–8.
Muntoni F, Wels D. Genetic treatments in muscular dystrophies. Curr Opin Neurol. 2007;20:590–4.
Kinali M, Arechavala-Gomeza V, Feng L, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–28.
Sookhoo S, Mackinnon I, Bushby K, Chinnery PF, Birchall D. MRI for the demonstration of subclinical muscle involvement in muscular dystrophy. Clin Radiol. 2007;62:160–5.
Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging. 2008;28:543–58.
Bernard CP, Liney GP, Manton DJ, Turnbull LW, Langton CM. Comparison of fat quantification methods: a phantom study at 3.0 T. J Magn Reson Imaging. 2008;27:192–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaeta, M., Messina, S., Mileto, A. et al. Muscle fat-fraction and mapping in Duchenne muscular dystrophy: evaluation of disease distribution and correlation with clinical assessments. Skeletal Radiol 41, 955–961 (2012). https://doi.org/10.1007/s00256-011-1301-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-011-1301-5