Abstract
Purpose
Synaptic abnormalities are associated with many brain disorders. Recently, we developed a novel synaptic vesicle glycoprotein 2A (SV2A) radiotracer [18F]SynVesT-1 and demonstrated its excellent imaging and binding properties in nonhuman primates. The aim of this study was to perform dosimetry calculations in nonhuman primates and to evaluate this tracer in humans and assess its test-retest reliability in comparison with [11C]UCB-J.
Methods
Three rhesus monkeys underwent whole body dynamic PET scanning to estimate the absorbed dose. PET scans in six healthy human subjects were acquired. Time-activity curves (TACs) were generated with defined regions of interest (ROI). Reproducibility of distribution volume (VT) values and its sensitivity to scan duration were assessed with the one-tissue compartment (1TC) model. Non-displaceable binding potential (BPND) was calculated using centrum semiovale as the reference region.
Results
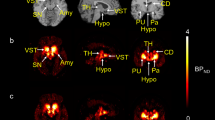
The dosimetry study showed high uptake in the urinary bladder and brain. In humans, [18F]SynVesT-1 displayed high uptake with maximum SUV of ~10 and appropriate kinetics with a quick rise in tracer uptake followed by a gradual clearance. Mean 1TC VT values (mL/cm3) ranged from 3.4 (centrum semiovale) to 19.6 (putamen) and were similar to those of [11C]UCB-J. Regional BPND values were 2.7–4.7 in gray matter areas, and mean BPND values across all ROIs were ~ 21% higher than those of [11C]UCB-J. The absolute test-retest variability of VT and BPND was excellent (< 9%) across all brain regions.
Conclusions
[18F]SynVesT-1 demonstrates outstanding characteristics in humans: fast and high brain uptake, appropriate tissue kinetics, high levels of specific binding, and excellent test-retest reproducibility of binding parameters. As such, [18F]SynVesT-1 is proved to be a favorable radiotracer for SV2A imaging and quantification in humans.



Similar content being viewed by others
References
Hamos JE, DeGennaro LJ, Drachman DA. Synaptic loss in Alzheimer’s disease and other dementia. Neurology. 1989;39:355–61. https://doi.org/10.1212/wnl.39.3.355.
Robinson JL, Molina-Porcel L, Corrada MM, Raible K, Lee EB, Lee VM, et al. Perforant path synaptic loss correlates with cognitive impairment and Alzheimer’s disease in the oldest-old. Brain. 2014;137:2578–87. https://doi.org/10.1093/brain/awu190.
Hou ZY, Lei H, Hong SH, Sun B, Fang K, Lin XT, et al. Functional changes in the frontal cortex in Parkinson's disease using a rat model. J Clin Neurosci. 2010;17:628–33. https://doi.org/10.1016/j.jocn.2009.07.101.
Proper EA, Oestreicher AB, Jansen GH, Veelen CW, van Rijen PC, Gispen WH, et al. Immunohistochemical characterization of mossy fibre sprouting in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain : a journal of neurology. 2000;123(Pt 1):19–30. https://doi.org/10.1093/brain/123.1.19.
Crevecoeur J, Kaminski RM, Rogister B, Foerch P, Vandenplas C, Neveux M, et al. Expression pattern of synaptic vesicle protein 2 (SV2) isoforms in patients with temporal lobe epilepsy and hippocampal sclerosis. Neuropathol Appl Neurobiol. 2014;40:191–204. https://doi.org/10.1111/nan.12054.
Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–7. https://doi.org/10.1038/nm.2886.
Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. https://doi.org/10.1001/archpsyc.57.1.65.
Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83. https://doi.org/10.1038/nature16549.
Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–43. https://doi.org/10.1016/j.neuron.2014.07.040.
Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci. 1994;14:5223–35. https://doi.org/10.1523/JNEUROSCI.14-09-05223.1994.
Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, et al. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc Natl Acad Sci U S A. 1999;96:15268–73. https://doi.org/10.1073/pnas.96.26.15268.
Yao J, Nowack A, Kensel-Hammes P, Gardner RG, Bajjalieh SM. Cotrafficking of SV2 and synaptotagmin at the synapse. J Neurosci. 2010;30:5569–78. https://doi.org/10.1523/JNEUROSCI.4781-09.2010.
Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A. 2004;101:9861–6. https://doi.org/10.1073/pnas.0308208101.
Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF, Chen MK, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra96. https://doi.org/10.1126/scitranslmed.aaf6667.
Nabulsi NB, Mercier J, Holden D, Carre S, Najafzadeh S, Vandergeten MC, et al. Synthesis and preclinical evaluation of 11C-UCB-J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med. 2016;57:777–84. https://doi.org/10.2967/jnumed.115.168179.
Finnema SJ, Nabulsi NB, Mercier J, Lin SF, Chen MK, Matuskey D, et al. Kinetic evaluation and test-retest reproducibility of [11C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab. 2018;38:2041–52. https://doi.org/10.1177/0271678x17724947.
Toyonaga T, Smith LM, Finnema SJ, Gallezot JD, Naganawa M, Bini J, et al. In vivo synaptic density imaging with 11C-UCB-J detects treatment effects of saracatinib (AZD0530) in a mouse model of Alzheimer’s disease. J Nucl Med. 2019;60:1780–6. https://doi.org/10.2967/jnumed.118.223867.
Chen MK, Mecca AP, Naganawa M, Finnema SJ, Toyonaga T, Lin SF, et al. Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA neurology. 2018;75:1215–24. https://doi.org/10.1001/jamaneurol.2018.1836.
Mecca AP, Chen MK, O'Dell RS, Naganawa M, Toyonaga T, Godek TA, et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimers Dement. 2020. https://doi.org/10.1002/alz.12097.
Matuskey D, Tinaz S, Wilcox KC, Naganawa M, Toyonaga T, Dias M, et al. Synaptic changes in Parkinson disease assessed with in vivo imaging. Ann Neurol. 2020;87:329–38. https://doi.org/10.1002/ana.25682.
Holmes SE, Scheinost D, Finnema SJ, Naganawa M, Davis MT, DellaGioia N, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun. 2019;10:1529. https://doi.org/10.1038/s41467-019-09562-7.
Onwordi EC, Halff EF, Whitehurst T, Mansur A, Cotel MC, Wells L, et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun. 2020;11:246. https://doi.org/10.1038/s41467-019-14122-0.
Li S, Cai Z, Wu X, Holden D, Pracitto R, Kapinos M, et al. Synthesis and in vivo evaluation of a novel PET radiotracer for imaging of synaptic vesicle glycoprotein 2A (SV2A) in nonhuman primates. ACS Chem Neurosci. 2019;10:1544–54. https://doi.org/10.1021/acschemneuro.8b00526.
Constantinescu CC, Tresse C, Zheng M, Gouasmat A, Carroll VM, Mistico L, et al. Development and in vivo preclinical imaging of Fluorine-18-labeled synaptic vesicle protein 2A (SV2A) PET tracers. Mol Imaging Biol. 2019;21:509–18. https://doi.org/10.1007/s11307-018-1260-5.
Cristy M, Eckerman KF, Marietta M, Systems E. (1987) Specific absorbed fractions of energy at various ages from internal photon sources. in ORNL Report ORNL/TM-8381 V1-V7 Oak Ridge, TN, Oak Ridge National Laboratory.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7.
Carson RE, Barker W, Liow J-S, Adler S, Johnson C. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction of the HRRT. IEEE Nucl Sci Symp Conf Rec. 2003:M16–6.
Jin X, Chan C, Mulnix T, Panin V, Casey ME, Liu C, et al. List-mode reconstruction for the biograph mCT with physics modeling and event-by-event motion correction. Phys Med Biol. 2013;58:5567–91. https://doi.org/10.1088/0031-9155/58/16/5567.
Hilton J, Yokoi F, Dannals RF, Ravert HT, Szabo Z, Wong DF. Column-switching HPLC for the analysis of plasma in PET imaging studies. Nucl Med Biol. 2000;27:627–30. https://doi.org/10.1016/S0969-8051(00)00125-6.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. https://doi.org/10.1006/nimg.2001.0978.
Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–33. https://doi.org/10.1097/00004728-199803000-00032.
Papademetris X, Jackowski M, Rajeevan N, Constable RT, Staib LH. Bioimage suite: an integrated medical image analysis suite. Insight J. 2005.
Rossano S, Toyonaga T, Finnema SJ, Naganawa M, Lu Y, Nabulsi N, et al. Assessment of a white matter reference region for 11C-UCB-J PET quantification. J Cereb Blood Flow Metab. 2020;40:1890–901. https://doi.org/10.1177/0271678X19879230.
Naganawa M, Li S, Nabulsi N, Henry S, Zheng M, Pracitto R, et al. First-in-human evaluation of 18F-SynVesT-1, a novel radioligand for PET imaging of synaptic vesicle protein 2A. J Nucl Med. 2020. https://doi.org/10.2967/jnumed.120.249144.
Quinn B, Dauer Z, Pandit-Taskar N, Schoder H, Dauer LT. Radiation dosimetry of 18F-FDG PET/CT: incorporating exam-specific parameters in dose estimates. BMC Med Imaging. 2016;16:41. https://doi.org/10.1186/s12880-016-0143-y.
Acknowledgments
The authors appreciate the excellent technical assistance of the staff at the Yale University PET Center. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Funding
This study was funded by the Michael J. Fox Foundation and National Institutes of Health (US) R01AG052560 & R01AG065474. This publication was also made possible by CTSA Grant UL1 RR024139 jointly from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS), components of the National Institutes of Health (NIH).
Author information
Authors and Affiliations
Contributions
Yiyun Huang and Richard E. Carson collectively contributed to the conception of the study and its design. Shanna Henry and David Matuskey were responsible for subject recruitment, evaluation, and care. Songye Li, Richard Pracitto, Soheila Najafzadeh, Paul R. Emory, Zhengxin Cai, Jim Ropchan, and Nabeel Nabulsi were responsible for tracer synthesis and quality control tests. Daniel Holden performed data analysis for the dosimetry study. Mika Naganawa performed analysis of imaging data in humans. Songye Li and Mika Naganawa drafted the manuscript. All authors participated in the editing of the manuscript and approved the manuscript and this submission.
Corresponding authors
Ethics declarations
Conflict of interest
The radioligand [18F]SynVesT-1 (formerly referred to as [18F]SDM-8) is contained in the international patent application PCT/US2018/018388, Radiolabeled Pharmaceuticals and Methods of Making and Using Same, filed on February 15, 2018 (Inventors: YH, ZC, SL, NN, and REC). The other authors declare that they have no conflict of interest.
Ethical approval
PET imaging experiments were performed in rhesus monkeys (Macaca mulatta) according to a protocol approved by the Yale University Institutional Animal Care and Use Committee. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
The study protocol involving human participants was approved by the Yale Human Investigation Committee, the Yale-New Haven Hospital Radiation Safety Committee, and the Yale University Radiation Safety Committee. Study procedures were performed in accordance with federal guidelines and regulations of the United States for the protection of human research subjects contained in Title 45 Part 46 of the Code of Federal Regulations (45 CFR 46).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Translational research.
Rights and permissions
About this article
Cite this article
Li, S., Naganawa, M., Pracitto, R. et al. Assessment of test-retest reproducibility of [18F]SynVesT-1, a novel radiotracer for PET imaging of synaptic vesicle glycoprotein 2A. Eur J Nucl Med Mol Imaging 48, 1327–1338 (2021). https://doi.org/10.1007/s00259-020-05149-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-05149-3