Abstract
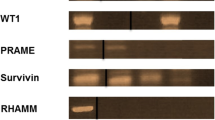
Melanoma is one of the most immunogenic tumors, and extensive lists of potential tumor rejection antigens have been collected during the last decades. By isolating human leukocyte antigen (HLA) class I complexes from five melanoma cell lines (FM-82, FM-93/2, Mel-624, MeWo and SK-Mel-5) and sequencing HLA-eluted peptides by mass spectrometry, we identified over 10,000 unique peptides with high confidence. The majority of the peptides were 8–11 amino acids in length and were predicted to bind to the respective HLA alleles. Over 250 epitopes, corresponding to previously described tumor-associated antigens, were identified, suggesting that HLA peptidome analysis may facilitate the characterization of putative tumor rejection antigens. MeWo and SK-Mel-5 cell lines were further interrogated for neo-epitopes, revealing one peptide from MeWo cells carrying an amino acid mutation. We also observed a remarkable overlap between A*03:01 peptides eluted from Mel-624 cells and A*03:01 peptides recovered from soluble HLA complexes purified from two melanoma patients, shedding light on the similarity of the HLA peptidome in cell lines and in patient-derived material. The reliable characterization of the HLA class I peptidome in melanoma promises to facilitate the identification of tumor rejection antigens and the development of immunotherapeutic strategies.




Similar content being viewed by others
Abbreviations
- ACN:
-
Acetonitrile
- ATCC:
-
American Type Culture Collection
- COSMIC:
-
Catalogue of Somatic Mutations in Cancer
- ESTDAB:
-
European Searchable Tumour Line Database
- ETH Zurich:
-
Eidgenössische Technische Hochschule Zürich
- FDR:
-
False discovery rate
- FPLC:
-
Fast protein liquid chromatography
- HCD:
-
Higher-energy collisional dissociation
- HLA:
-
Human leukocyte antigen
- IC50 :
-
Half maximal inhibitory concentration
- LC-MS:
-
Liquid chromatography–mass spectrometry
- MS:
-
Mass spectrometry
- MS/MS:
-
Fragment mass spectra
- PMSF:
-
Phenylmethylsulfonyl fluoride
- sHLA:
-
Soluble human leukocyte antigen
- UHPLC:
-
Ultra high performance liquid chromatography
- WT:
-
Wild type
References
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264. doi:10.1038/nrc3239
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348(6230):56–61. doi:10.1126/science.aaa8172
Probst P, Neri D (2014) Immunocytokines for cancer therapy. Forum Immunopathol Dis Therap 5(1):83–99. doi:10.1615/ForumImmunDisTher.2015014013
Kontermann RE, Brinkmann U (2015) Bispecific antibodies. Drug Discov Today 20(7):838–847. doi:10.1016/j.drudis.2015.02.008
Rosenberg SA, Restifo NP (2015) Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348(6230):62–68. doi:10.1126/science.aaa4967
June CH, Riddell SR, Schumacher TN (2015) Adoptive cellular therapy: a race to the finish line. Sci Transl Med 7(280):280ps7. doi:10.1126/scitranslmed.aaa3643
Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, Mellstedt H (2014) Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol 11(9):509–524. doi:10.1038/nrclinonc.2014.111
Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, Samuels Y, Rosenberg SA (2013) Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 19(6):747–752. doi:10.1038/nm.3161
Scheibenbogen C, Sun Y, Keilholz U, Song M, Stevanovic S, Asemissen AM, Nagorsen D, Thiel E, Rammensee HG, Schadendorf D (2002) Identification of known and novel immunogenic T-cell epitopes from tumor antigens recognized by peripheral blood T cells from patients responding to IL-2-based treatment. Int J Cancer 98(3):409–414
Andersen RS, Thrue CA, Junker N, Lyngaa R, Donia M, Ellebaek E, Svane IM, Schumacher TN, Thor Straten P, Hadrup SR (2012) Dissection of T-cell antigen specificity in human melanoma. Cancer Res 72(7):1642–1650. doi:10.1158/0008-5472.CAN-11-2614
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjord JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jager N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, Lopez-Otin C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdes-Mas R, van Buuren MM, van’t Veer L, Vincent-Salomon A, Waddell N, Yates LR, PedBrain I, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR, Australian Pancreatic Cancer Genome I, Consortium IBC, Consortium IM-S (2013) Signatures of mutational processes in human cancer. Nature 500(7463):415–421. doi:10.1038/nature12477
Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW (2013) Cancer genome landscapes. Science 339(6127):1546–1558. doi:10.1126/science.1235122
Hadrup SR, Schumacher TN (2010) MHC-based detection of antigen-specific CD8+ T cell responses. Cancer Immunol Immunother 59(9):1425–1433. doi:10.1007/s00262-010-0824-2
Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM (1996) Phenotypic analysis of antigen-specific T lymphocytes. Science 274(5284):94–96
Tran E, Ahmadzadeh M, Lu YC, Gros A, Turcotte S, Robbins PF, Gartner JJ, Zheng Z, Li YF, Ray S, Wunderlich JR, Somerville RP, Rosenberg SA (2015) Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 350(6266):1387–1390. doi:10.1126/science.aad1253
Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJ, van der Burg S, Kapiteijn E, Michielin O, Romano E, Linnemann C, Speiser D, Blank C, Haanen JB, Schumacher TN (2014) Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med 6(254):254ra128. doi:10.1126/scitranslmed.3008918
Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL Jr (1994) Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science 264(5159):716–719
Jarmalavicius S, Welte Y, Walden P (2012) High immunogenicity of the human leukocyte antigen peptidomes of melanoma tumor cells. J Biol Chem 287(40):33401–33411. doi:10.1074/jbc.M112.358903
Pritchard AL, Hastie ML, Neller M, Gorman JJ, Schmidt CW, Hayward NK (2015) Exploration of peptides bound to MHC class I molecules in melanoma. Pigment Cell Melanoma Res 28(3):281–294. doi:10.1111/pcmr.12357
Hassan C, Kester MG, de Ru AH, Hombrink P, Drijfhout JW, Nijveen H, Leunissen JA, Heemskerk MH, Falkenburg JH, van Veelen PA (2013) The human leukocyte antigen-presented ligandome of B lymphocytes. Mol Cell Proteom 12(7):1829–1843. doi:10.1074/mcp.M112.024810
Bassani-Sternberg M, Pletscher-Frankild S, Jensen LJ, Mann M (2015) Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation. Mol Cell Proteom 14(3):658–673. doi:10.1074/mcp.M114.042812
Ritz D, Gloger A, Weide B, Garbe C, Neri D, Fugmann T (2016) High-sensitivity HLA class I peptidome analysis enables a precise definition of peptide motifs and the identification of peptides from cell lines and patients’ sera. Proteomics 16(10):1570–1580. doi:10.1002/pmic.201500445
Pawelec G, Marsh SG (2006) ESTDAB: a collection of immunologically characterised melanoma cell lines and searchable databank. Cancer Immunol Immunother 55(6):623–627. doi:10.1007/s00262-005-0117-3
Chou J, Voong LN, Mortales CL, Towlerton AM, Pollack SM, Chen X, Yee C, Robbins PF, Warren EH (2012) Epigenetic modulation to enable antigen-specific T-cell therapy of colorectal cancer. J Immunother 35(2):131–141. doi:10.1097/CJI.0b013e31824300c7
Toebes M, Rodenko B, Ovaa H, Schumacher TN (2009) Generation of peptide MHC class I monomers and multimers through ligand exchange. Curr Protoc Immunol. doi:10.1002/0471142735.im1816s87
Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26(12):1367–1372. doi:10.1038/nbt.1511
Nielsen M, Lundegaard C, Blicher T, Lamberth K, Harndahl M, Justesen S, Roder G, Peters B, Sette A, Lund O, Buus S (2007) NetMHCpan, a method for quantitative predictions of peptide binding to any HLA-A and -B locus protein of known sequence. PLoS One 2(8):e796. doi:10.1371/journal.pone.0000796
Boegel S, Lower M, Bukur T, Sahin U, Castle JC (2014) A catalog of HLA type, HLA expression, and neo-epitope candidates in human cancer cell lines. Oncoimmunology 3(8):e954893. doi:10.4161/21624011.2014.954893
Thomsen MC, Nielsen M (2012) Seq2Logo: a method for construction and visualization of amino acid binding motifs and sequence profiles including sequence weighting, pseudo counts and two-sided representation of amino acid enrichment and depletion. Nucleic Acids Res 40(Web Server issue):W281–W287. doi:10.1093/nar/gks469
Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, Teague JW, Campbell PJ, Stratton MR, Futreal PA (2011) COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res 39(Database issue):D945–D950. doi:10.1093/nar/gkq929
Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A (1978) Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell 14(1):9–20
Rapin N, Hoof I, Lund O, Nielsen M (2008) MHC motif viewer. Immunogenetics 60(12):759–765. doi:10.1007/s00251-008-0330-2
Rasmussen M, Harndahl M, Stryhn A, Boucherma R, Nielsen LL, Lemonnier FA, Nielsen M, Buus S (2014) Uncovering the peptide-binding specificities of HLA-C: a general strategy to determine the specificity of any MHC class I molecule. J Immunol 193(10):4790–4802. doi:10.4049/jimmunol.1401689
Apps R, Meng Z, Del Prete GQ, Lifson JD, Zhou M, Carrington M (2015) Relative expression levels of the HLA class-I proteins in normal and HIV-infected cells. J Immunol 194(8):3594–3600. doi:10.4049/jimmunol.1403234
Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA (2014) Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371(23):2189–2199. doi:10.1056/NEJMoa1406498
Schumacher TN, Schreiber RD (2015) Neoantigens in cancer immunotherapy. Science 348(6230):69–74. doi:10.1126/science.aaa4971
Weide B, Zelba H, Derhovanessian E, Pflugfelder A, Eigentler TK, Di Giacomo AM, Maio M, Aarntzen EH, de Vries IJ, Sucker A, Schadendorf D, Buttner P, Garbe C, Pawelec G (2012) Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol 30(15):1835–1841. doi:10.1200/JCO.2011.40.2271
Haen SP, Rammensee HG (2013) The repertoire of human tumor-associated epitopes–identification and selection of antigens and their application in clinical trials. Curr Opin Immunol 25(2):277–283. doi:10.1016/j.coi.2013.03.007
Acknowledgments
We thank Camilla Bacci (Philogen SpA) for providing W6/32 antibody.
Funding
This work was supported financially by ETH Zürich, the Swiss National Science Foundation, the European Union’s Seventh Framework Programme (FP7/2007-2013) under Grant Agreement Nos. 305309 (PRIAT) and 305608 (EURenOmics), the Bonveda Foundation and the European Research Council (ERC advanced Grant “ZAUBERKUGEL”).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Dario Neri is co-founder of Philogen, shareholder and member of the board. Tim Fugmann and Danilo Ritz are employees of Philochem AG. The authors declare no additional conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gloger, A., Ritz, D., Fugmann, T. et al. Mass spectrometric analysis of the HLA class I peptidome of melanoma cell lines as a promising tool for the identification of putative tumor-associated HLA epitopes. Cancer Immunol Immunother 65, 1377–1393 (2016). https://doi.org/10.1007/s00262-016-1897-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1897-3