Abstract
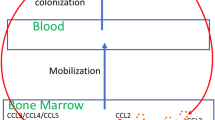
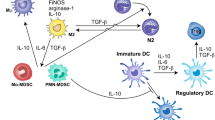
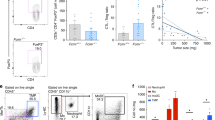
Malignant melanoma is characterized by the development of chronic inflammation in the tumor microenvironment, leading to the accumulation of myeloid-derived suppressor cells (MDSCs). Using ret transgenic mouse melanoma model, we found a significant migration of MDSCs expressing C-C chemokine receptor (CCR)5 into primary tumors and metastatic lymph nodes, which was correlated with tumor progression. An increased CCR5 expression on MDSCs was associated with elevated concentrations of CCR5 ligands in melanoma microenvironment. In vitro experiments showed that the upregulation of CCR5 expression on CD11b+Gr1+ immature myeloid cells was induced by CCR5 ligands, IL-6, GM-CSF, and other inflammatory factors. Furthermore, CCR5+ MDSCs infiltrating melanoma lesions displayed a stronger immunosuppressive pattern than their CCR5− counterparts. Targeting CCR5/CCR5 ligand signaling via a fusion protein mCCR5-Ig, which selectively binds and neutralizes all three CCR5 ligands, increased the survival of tumor-bearing mice. This was associated with a reduced migration and immunosuppressive potential of tumor MDSCs. In melanoma patients, circulating CCR5+ MDSCs were increased as compared to healthy donors. Like in melanoma-bearing mice, we observed an enrichment of these cells and CCR5 ligands in tumors as compared to the peripheral blood. Our findings define a critical role for CCR5 not only in the recruitment but also in the activation of MDSCs in tumor lesions, suggesting that novel strategies of melanoma treatment could be based on blocking CCR5/CCR5 ligand interactions.


Similar content being viewed by others
Abbreviations
- ARG:
-
Arginase
- BM:
-
Bone marrow
- CCL:
-
C-C motif ligand
- CCR:
-
C-C motif receptor
- CXCL:
-
C-X-C motif ligand
- CXCR:
-
C-X-C motif receptor
- DCs:
-
Dendritic cells
- GM–CSF:
-
Granulocyte–macrophage colony-stimulating factor
- HIF:
-
Hypoxia-inducible factor
- IFN:
-
Interferon
- IL:
-
Interleukin
- M:
-
Monocytic
- MDSCs:
-
Myeloid-derived suppressor cells
- NF-κB:
-
Nuclear factor-κB
- NO:
-
Nitric oxide
- PD-1:
-
Programmed death receptor
- PD-L1:
-
Programmed death-ligand 1
- PMN:
-
Polymorphonuclear
- ROS:
-
Reactive oxygen species
- TGF:
-
Transforming growth factor
- TNF:
-
Tumor necrosis factor
- Tregs:
-
Regulatory T cells
- VEGF:
-
Vascular endothelial growth factor
References
Eggermont AM, Spatz A, Robert C (2014) Cutaneous melanoma. Lancet 383:816–827
Stadler S, Weina K, Gebhardt C, Utikal J (2015) New therapeutic options for advanced non-resectable malignant melanoma. Adv Med Sci 60:83–88
Gogas H, Polyzos A, Kirkwood J (2013) Immunotherapy for advanced melanoma: fulfilling the promise. Cancer Treat Rev 39:879–885
Rosenberg SA, Restifo NP (2015) Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348:62–68
Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint blockade in cancer therapy. J Clin Oncol 33:1974–1982
Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, Holland-Letz T, Umansky L, Beckhove P, Sucker A, Schadendorf D, Utikal J, Umansky V (2015) Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res 21:5453–5459
Umansky V, Sevko A, Gebhardt C, Utikal J (2014) Myeloid-derived suppressor cells in malignant melanoma. J Dtsch Dermatol Ges 12:1021–1027
Zimmer L, Eigentler TK, Kiecker F, Simon J, Utikal J, Mohr P, Berking C, Kämpgen E, Dippel E, Stadler R, Hauschild A, Fluck M, Terheyden P, Rompel R, Loquai C, Assi Z, Garbe C, Schadendorf D (2015) Open-label, multicenter, single-arm phase II DeCOG-study of ipilimumab in pretreated patients with different subtypes of metastatic melanoma. J Transl Med 13:351
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V (2012) Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12:253–268
Parker KH, Beury DW, Ostrand-Rosenberg S (2015) Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res 128:95–139
Kanterman J, Sade-Feldman M, Baniyash M (2012) New insights into chronic inflammation-induced immunosuppression. Semin Cancer Biol 22:307–318
Umansky V, Sevko A (2012) Overcoming immunosuppression in the melanoma microenvironment induced by chronic inflammation. Cancer Immunol Immunother 61:275–282
Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI (2016) Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7:12150
Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V (2014) Myeloid-derived suppressor cell heterogeneity in human cancers. Ann NY Acad Sci 1319:47–65
Filipazzi P, Huber V, Rivoltini L (2012) Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother 61:255–263
Ostrand-Rosenberg S (2010) Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother 59:1593–1600
Homey B, Muller A, Zlotnik A (2002) Chemokines: agents for the immunotherapy of cancer?. Nat Rev Immunol 2:175–184.
Combadiere C, Ahuja SK, Tiffany HL, Murphy PM (1996) Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1(alpha), MIP-1(beta), and RANTES. J Leukoc Biol 60:147–152
Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, Rizzuto GA, Lazarus JJ, Pamer EG, Houghton AN, Merghoub T, Wolchok JD (2012) Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res 72:876–886
Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, Matsushima K (2008) Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 111:5457–5466
Izhak L, Wildbaum G, Zohar Y, Anunu R, Klapper L, Elkeles A, Seagal J, Yefenof E, Ayalon-Soffer M, Karin N (2009) A novel recombinant fusion protein encoding a 20-amino acid residue of the third extracellular (E3) domain of CCR2 neutralizes the biological activity of CCL2. J Immunol 183:732–739
Izhak L, Wildbaum G, Weinberg U, Shaked Y, Alami J, Dumont D, Friedman B, Stein A, Karin N (2010) Predominant expression of CCL2 at the tumor site of prostate cancer patients directs a selective loss of immunological tolerance to CCL2 that could be amplified in a beneficial manner. J Immunol 184:1092–1101
Izhak L, Wildbaum G, Jung S, Stein A, Shaked Y, Karin N (2012) Dissecting the autocrine and paracrine roles of the CCR2–CCL2 axis in tumor survival and angiogenesis. PLoS ONE 7:e28305
Ugel S, De Sanctis F, Mandruzzato S, Bronte V (2015) Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Investig 125:3365–3376
Kalinski P (2012) Regulation of immune responses by prostaglandin E2. J Immunol 188:21–28
Williams SA, Harata-Lee Y, Comerford I, Anderson RL, Smyth MJ, McColl SR (2010) Multiple functions of CXCL12 in a syngeneic model of breast cancer. Mol Cancer 9:250
Connolly MK, Mallen-St Clair J, Bedrosian AS, Malhotra A, Vera V, Ibrahim J, Henning J, Pachter HL, Bar-Sagi D, Frey AB, Miller G (2010) Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and preinvasive intra-abdominal tumor. J Leukoc Biol 87:713–725
Wang SW, Liu SC, Sun HL, Huang TY, Chan CH, Yang CY, Yeh HI, Huang YL, Chou WY, Lin YM, Tang CH (2015) CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis 36:104–114
Appay V, Rowland-Jones SL (2001) RANTES: a versatile and controversial chemokine. Trends Immunol 22:83–87
Harper AR, Nayee S, Topol EJ (2015) Protective alleles and modifier variants in human health and disease. Nat Rev Genet 16:689–701
Balistreri CR, Carruba G, Calabrò M, Campisi I, Di Carlo D, Lio D, Colonna-Romano G, Candore G, Caruso C (2009) CCR5 proinflammatory allele in prostate cancer risk: a pilot study in patients and centenarians from Sicily. Ann NY Acad Sci 1155:289–292
Velasco-Velazquez M, Jiao X, De La Fuente M, Pestell TG, Ertel A, Lisanti MP, Pestell RG (2012) CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res 72:3839–3850
Song JK, Park MH, Choi DY, Yoo HS, Han SB, Yoon DY, Hong JT (2012) Deficiency of C-C chemokine receptor 5 suppresses tumor development via inactivation of NF-kappaB and upregulation of IL-1Ra in melanoma model. PLoS ONE 7:e33747
Mencarelli A, Graziosi L, Renga B, Cipriani S, D’Amore C, Francisci D, Bruno A, Baldelli F, Donini A, Fiorucci S (2013) CCR5 antagonism by maraviroc reduces the potential for gastric cancer cell dissemination. Transl Oncol 6:784–793
Sicoli D, Jiao X, Ju X, Velasco-Velazquez M, Ertel A, Addya S, Li Z, Andò S, Fatatis A, Paudyal B, Cristofanilli M, Thakur ML, Lisanti MP, Pestell RG (2014) CCR5 receptor antagonists block metastasis to bone of v-Src oncogene-transformed metastatic prostate cancer cell lines. Cancer Res 74:7103–7114
Che LF, Shao SF, Wang LX (2016) Downregulation of CCR5 inhibits the proliferation and invasion of cervical cancer cells and is regulated by microRNA-107. Exp Ther Med 11:503–509.
van Deventer HW, O’Connor W Jr, Brickey WJ, Aris RM, Ting JP, Serody JS (2005) C-C chemokine receptor 5 on stromal cells promotes pulmonary metastasis. Cancer Res 65:3374–3379
Ng-Cashin J, Kuhns JJ, Burkett SE, Powderly JD, Craven RR, van Deventer HW, Kirby SL, Serody JS (2003) Host absence of CCR5 potentiates dendritic cell vaccination. J Immunol 170:4201–4208
Aldinucci D, Colombatti A (2014) The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm 2014:292376
Chang LY, Lin YC, Kang CW, Hsu CY, Chu YY, Huang CT, Day YJ, Chen TC, Yeh CT, Lin CY (2012) The indispensable role of CCR5 for in vivo suppressor function of tumor-derived CD103+ effector/memory regulatory T cells. J Immunol 189:567–574
Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, Cerwenka A (2012) Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol 189:5602–5611
Umansky V, Abschuetz O, Osen W, Ramacher M, Zhao F, Kato M, Schadendorf D (2008) Melanoma-specific memory T cells are functionally active in Ret transgenic mice without macroscopic tumors. Cancer Res 68:9451–9458
Zhu Z, Aref AR, Cohoon TJ, Barbie TU, Imamura Y, Yang S, Moody SE, Shen RR, Schinzel AC, Thai TC, Reibel JB, Tamayo P, Godfrey JT, Qian ZR, Page AN, Maciag K, Chan EM, Silkworth W, Labowsky MT, Rozhansky L, Mesirov JP, Gillanders WE, Ogino S, Hacohen N, Gaudet S, Eck MJ, Engelman JA, Corcoran RB, Wong KK, Hahn WC, Barbie DA (2014) Inhibition of KRAS-driven tumorigenicity by interruption of an autocrine cytokine circuit. Cancer Discov 4:452–465.
Richmond A, Yang J, Su Y (2009) The good and the bad of chemokines/chemokine receptors in melanoma. Pigment Cell Melanoma Res 22:175–186
Gao D, Rahbar R, Fish EN (2016) CCL5 activation of CCR5 regulates cell metabolism to enhance proliferation of breast cancer cells. Open Biol 6:160122
Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M, Umansky V (2011) Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci USA 108:17111–17116
Sevko A, Michels T, Vrohlings M, Umansky L, Beckhove P, Kato M, Shurin GV, Shurin MR, Umansky V (2013) Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol 190:2464–2471
Lin S, Wan S, Sun L, Hu J, Fang D, Zhao R, Yuan S, Zhang L (2012) Chemokine C-C motif receptor 5 and C-C motif ligand 5 promote cancer cell migration under hypoxia. Cancer Sci 103:904–912
Zhang Y, Lv D, Kim HJ, Kurt RA, Bu W, Li Y, Ma X (2013) A novel role of hematopoietic CCL5 in promoting triple-negative mammary tumor progression by regulating generation of myeloid-derived suppressor cells. Cell Res 23:394–408
Ward ST, Li KK, Hepburn E, Weston CJ, Curbishley SM, Reynolds GM, Hejmadi RK, Bicknell R, Eksteen B, Ismail T, Rot A, Adams DH (2015) The effects of CCR5 inhibition on regulatory T-cell recruitment to colorectal cancer. Br J Cancer 112:319–328
Ray N (2009) Maraviroc in the treatment of HIV infection. Drug Des Dev Ther 2:151–161
Saita Y, Kondo M, Shimizu Y (2007) Species selectivity of small-molecular antagonists for the CCR5 chemokine receptor. Int Immunopharmacol 7:1528–1534
Pervaiz A, Ansari S, Berger MR, Adwan H (2015) CCR5 blockage by maraviroc induces cytotoxic and apoptotic effects in colorectal cancer cells. Med Oncol 32:158
Halama N, Zoernig I, Berthel A, Kahlert C, Klupp F, Suarez-Carmona M, Suetterlin T, Brand K, Krauss J, Lasitschka F, Lerchl T, Luckner-Minden C, Ulrich A, Koch M, Weitz J, Schneider M, Buechler MW, Zitvogel L, Herrmann T, Benner A, Kunz C, Luecke S, Springfeld C, Grabe N, Falk CS, Jaeger D (2016) Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell 29:587–601
Tang Q, Jiang J, Liu J (2015) CCR5 blockade suppresses melanoma development through inhibition of IL-6-Stat3 pathway via upregulation of SOCS3. Inflammation 38:2049–2056
Chang LY, Lin YC, Mahalingam J, Huang CT, Chen TW, Kang CW, Peng HM, Chu YY, Chiang JM, Dutta A, Day YJ, Chen TC, Yeh CT, Lin CY (2012) Tumor-derived chemokine CCL5 enhances TGF-beta-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res 72:1092–1102
Sapir Y, Vitenshtein A, Barsheshet Y, Zohar Y, Wildbaum G, Karin N (2010) A fusion protein encoding the second extracellular domain of CCR5 arrests chemokine-induced cosignaling and effectively suppresses ongoing experimental autoimmune encephalomyelitis. J Immunol 185:2589–2599
Jordan KR, Amaria RN, Ramirez O, Callihan EB, Gao D, Borakove M, Manthey E, Borges VF, McCarter MD (2013) Myeloid-derived suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol Immunother 62:1711–1722
Weide B, Martens A, Zelba H, Derhovanessian E, Bailur JK, Kyzirakos C, Pflugfelder A, Eigentler TK, Di Giacomo AM, Maio M, Aarntzen EH, de Vries J, Sucker A, Schadendorf D, Büttner P, Garbe C, Pawelec G (2014) Myeloid-derived suppressor cells predict survival of advanced melanoma patients: comparison with regulatory T cells and NY-ESO-1- or Melan-A-specific T cells. Clin Cancer Res 20:1601–1609
Pico de Coaña Y, Poschke I, Gentilcore G, Mao Y, Nyström M, Hansson J, Masucci GV, Kiessling R (2013) Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production. Cancer Immunol Res 1:158–162
Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, Zhu J, Wei H, Zhao K (2013) Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS ONE 8:e57114
Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, Garrett-Mayer E, Montero AJ, Bronte V, Mandruzzato S (2011) A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood 118:2254–2265
Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, Umansky V (2015) Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer 136:2352–2360
Payne AS, Cornelius LA (2002) The role of chemokines in melanoma tumor growth and metastasis. J Investig Dermatol 118:915–922
Acknowledgements
This work was supported by Grants from the German Research Council (RTG2099 to J. Utikal, V. Umansky and GE-2152/1-2 to C. Gebhardt), the Cooperation between German Cancer Research Center (DKFZ) and Ministry of Science, Technology and Space of Israel (MOST) in Cancer Research (CA157 to V. Umansky and C. Blattner) and the German Cancer Aid (109312 to J. Utikal). This work was kindly backed by the COST Action “European Network of Investigators Triggering Exploratory Research on Myeloid Regulatory Cells” (Mye-EUNITER). COST is supported by the EU Framework Program Horizon 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This paper is a Focussed Research Review based on a presentation given at the conference Regulatory Myeloid Suppressor Cells: From Basic Discovery to Therapeutic Application which was hosted by the Wistar Institute in Philadelphia, PA, USA, 16th–19th June, 2016. It is part of a Cancer Immunology, Immunotherapy series of Focussed Research Reviews.
Rights and permissions
About this article
Cite this article
Umansky, V., Blattner, C., Gebhardt, C. et al. CCR5 in recruitment and activation of myeloid-derived suppressor cells in melanoma. Cancer Immunol Immunother 66, 1015–1023 (2017). https://doi.org/10.1007/s00262-017-1988-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-1988-9