Abstract
Purpose
Circularly permuted TRAIL (CPT) has exhibited promising efficacy as a mono-therapy or in combination with thalidomide for patients with multiple myeloma (MM). In this phase 2 study, the safety and efficacy of CPT in combination with thalidomide and dexamethasone (CPT + TD) was evaluated in patients with pretreated relapsed/refractory MM (RRMM).
Methods
Patients who received at least two previous therapies for MM were randomly assigned at a 2:1 ratio to receive treatment with CPT + TD or thalidomide and dexamethasone (TD). The primary endpoint was the overall response rate (ORR), and the secondary endpoints included progression-free survival (PFS), duration of response (DOR) and safety.
Results
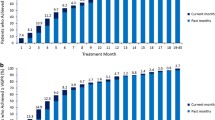
Overall, 47 patients were assigned to the CPT + TD group, and 24 patients were recruited to the TD group. The ORR in the CPT + TD group was 38.3 vs. 25.0% in the TD group. The median PFS time was 6.7 months for the CPT + TD group and 3.1 months for the TD group. The median DORs for the CPT + TD and TD groups were 7.1 and 3.2 months, respectively. Most of the adverse effects (AEs) were grade 1 or 2. Serious AEs were reported in 19.7% of the patients. No treatment-related deaths were reported.
Conclusion
CPT plus TD could serve as a new therapeutic strategy for patients with RRMM. A randomized, double-blind, placebo-controlled confirmatory study is currently under way.



Similar content being viewed by others
References
Landgren O, Iskander K (2017) Modern multiple myeloma therapy: deep, sustained treatment response and good clinical outcomes. J Intern Med 281(4):365–382. doi:10.1111/joim.12590
Kazandjian D, Landgren O (2016) A look backward and forward in the regulatory and treatment history of multiple myeloma: approval of novel-novel agents, new drug development, and longer patient survival. Semin Oncol 43(6):682–689. doi:10.1053/j.seminoncol.2016.10.008
Fang F, Wang AP, Yang SF (2005) Antitumor activity of a novel recombinant mutant human tumor necrosis factor-related apoptosis-inducing ligand. Acta Phamacologica Sinica 26:1373–1381. doi:10.1111/j.1745-7254.2005.00206.x
Zhou H, Li J, Jian Y et al (2016) Effects and mechanism of arsenic trioxide in combination with rmhTRAIL in multiple myeloma. Exp Hematol 44:125–131. doi:10.1016/j.exphem.2015.10.004
Yan D, Ge Y, Deng H et al (2015) Gefitinib upregulates death receptor 5 expression to mediate rmhTRAIL-induced apoptosis in Gefitinib-sensitive NSCLC cell line. Onco Targets Ther 8:1603–1610. doi:10.2147/OTT.S73731
Kelley SK, Harris LA, Xie D et al (2001) Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther 299:31–38
Chen WM, Qiu LG, Hou J et al (2012) Phase Ib study of recombinant circularly permuted TRAIL (CPT) in relapsed or refractory multiple myeloma patients. Blood 120:1857 (ASH annual meeting abstracts)
Leng Y, Qiu L, Hou J et al (2016) Phase II open-label study of recombinant circularly permuted TRAIL as a single-agent treatment for relapsed or refractory multiple myeloma. Chin J Cancer 35:86. doi:10.1186/s40880-016-0140-0
Geng C, Hou J, Zhao Y et al (2014) A multicenter, open-label phase II study of recombinant CPT (circularly permuted TRAIL) plus thalidomide in patients with relapsed and refractory multiple myeloma. Am J Hematol 89:1037–1042. doi:10.1002/ajh.23822
Anderson KC, Alsina M, Bensinger W et al (2013) Multiple myeloma, version 1, 2013. J Natl Compr Canc Netw 11:11–17
Chen W (2016) The guidelines for the diagnosis and management of multiple myeloma in China (2015 revision): interpretation of the treatment of relapsing and refractory multiple myeloma. Zhonghua Nei Ke Za Zhi 55:93–94. doi:10.3760/cma.j.issn.0578-1426.2016.02.004
Bladé J, Samson D, Reece D et al (1998) Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol 102:1115–1123. doi:10.1046/j.1365-2141.1998.00930.x
Durie BG, Harousseau JL, Miguel JS, International Myeloma Working Group et al (2006) International uniform response criteria for multiple myeloma. Leukemia 20:1467–1473. doi:10.1038/sj.leu.2404284
Palumbo A, Giaccone L, Bertola A et al (2001) Low-dose thalidomide plus dexamethasone is an effective salvage therapy for advanced myeloma. Haematologica 86:399–403
Dimopoulos MA, Zervas K, Kouvatseas G et al (2001) Thalidomide and dexamethasone combination for refractory multiple myeloma. Ann Oncol 12:991–995. doi:10.1023/A:1011132808904
Anagnostopoulos A, Weber D, Rankin K et al (2003) Thalidomide and dexamethasone for resistant multiple myeloma. Br J Haematol 121:768–771. doi:10.1046/j.1365-2141.2003.04345.x
Chen WM, Wei P, Yang SF et al (2014) Transiently elevated AST/LDH are associated with clinical response to recombinant circularly permuted TRAIL (CPT) plus thalidomide in patients with relapsed and/or refractory multiple myeloma. Blood 124:3478 (ASH annual meeting abstracts)
Wei P, Yang S, Zheng X et al (2014) Transient elevation of AST or LDH as an early pharmacodynamic biomarker of recombinant circularly permuted trail (CPT) in treating patients with relapsed or refractory multiple myeloma. Haematologica 99:369 (19th congress of EHA Abstracts, Abstract P974)
Acknowledgements
The authors would like to thank all the patients and their families who contributed to the present study. The authors would like to thank all the staff members from the participating study sites.
Author information
Authors and Affiliations
Contributions
Wenming Chen, Jian Hou, Shifang Yang and Xiangjun Zheng conceived, designed and performed the study; Yun Leng, Jian Hou, Jie Jin, Mei Zhang, Xiaoyan Ke, Bin Jiang, Ling Pan, Linhua Yang, Fang Zhou, Jianmin Wang, Zhao Wang, Li Liu, Wei Li, Zhixiang Shen, Lugui Qiu, Naibai Chang, Jianyong Li, Jing Liu, Haitao Meng, Hua Jiang, Yan Liu and Wenming Chen enrolled patients and collected data; Wenming Chen, Jian Hou, Shifang Yang, Xiangjun Zheng, Peng Wei and Hongyan Pang analyzed and interpreted the data; and all authors critically reviewed the manuscript for important intellectual content and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
Beijing Sunbio Biotech Co. Ltd sponsored this clinical study and was responsible for medical monitoring and auditing. Shifang Yang, Xiangjun Zheng, Peng Wei and Hongyan Pang are employees of Beijing Sunbio Biotech Co., Ltd. All of the other authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
This trial was registered at http://www.chictr.org as ChiCTR-TRC-11001625.
Rights and permissions
About this article
Cite this article
Leng, Y., Hou, J., Jin, J. et al. Circularly permuted TRAIL plus thalidomide and dexamethasone versus thalidomide and dexamethasone for relapsed/refractory multiple myeloma: a phase 2 study. Cancer Chemother Pharmacol 79, 1141–1149 (2017). https://doi.org/10.1007/s00280-017-3310-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3310-0