Abstract
Background
Angiogenesis, the formation of blood vessel, is required for invasive tumor growth and metastasis. In this study, the effects of lidocaine, an amide-link local anesthetic, on angiogenesis and tumor growth were investigated.
Methods
In vitro angiogenesis assays were conducted using human umbilical vascular endothelial cell (HUVEC). Essential molecules involved in vascular endothelial growth factor (VEGF) signaling were analyzed. Tumor angiogenesis was analyzed using in vivo mouse tumor model.
Results
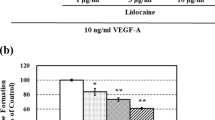
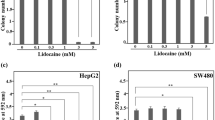
Lidocaine at clinically relevant concentrations inhibited angiogenesis. Lidocaine inhibited endothelial cell in vitro capillary network formation on Matrigel through interfering early stage of angiogenesis. In addition, lidocaine inhibited vascular endothelial growth factor (VEGF)-stimulated endothelial cell migration and proliferation without affecting cell adhesion. Lidocaine also induced endothelial cell apoptosis in the presence of VEGF. Lidocaine suppressed VEGF-activated phosphorylation of VEGF receptor 2 (VEGFR2), PLCγ-PKC-MAPK and FAK-paxillin in endothelial cells, demonstrating that VEGF, PLC, MAPK and FAK-paxillin suppression is associated with the antiangiogenic effect of lidocaine. Importantly, the in vitro observations were translatable to in vivo B16 melanoma mouse model. Lidocaine significantly inhibited tumor angiogenesis, leading to delay of tumor growth.
Conclusions
This study is the first to report that lidocaine acts as an angiogenesis inhibitor. The findings provide preclinical evidence into the potential mechanisms by which lidocaine may negatively affect cancer growth and metastasis.





Similar content being viewed by others
References
Zhang W, Liu JN, Tan XY (2009) Vaccination with xenogeneic tumor endothelial proteins isolated in situ inhibits tumor angiogenesis and spontaneous metastasis. Int J Cancer 125(1):124–132. https://doi.org/10.1002/ijc.24362
Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T, Horiuchi T, Muraoka R, Iki M (1996) Extent of tumor vascularization correlates with prognosis and hematogenous metastasis in gastric carcinomas. Cancer Res 56(11):2671–2676
Sarbia M, Bittinger F, Porschen R, Dutkowski P, Willers R, Gabbert HE (1996) Tumor vascularization and prognosis in squamous cell carcinomas of the esophagus. Anticancer Res 16(4A):2117–2121
Shibuya M (2011) Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2(12):1097–1105. https://doi.org/10.1177/1947601911423031
Koch S, Claesson-Welsh L (2012) Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med 2(7):a006502. https://doi.org/10.1101/cshperspect.a006502
Keating GM (2014) Bevacizumab: a review of its use in advanced cancer. Drugs 74(16):1891–1925. https://doi.org/10.1007/s40265-014-0302-9
Nassif E, Thibault C, Vano Y, Fournier L, Mauge L, Verkarre V, Timsit MO, Mejean A, Tartour E, Oudard S (2017) Sunitinib in kidney cancer: 10 years of experience and development. Expert Rev Anticancer Ther 17(2):129–142. https://doi.org/10.1080/14737140.2017.1272415
Poveda A, del Muro XG, Lopez-Guerrero JA, Martinez V, Romero I, Valverde C, Cubedo R, Martin-Broto J (2014) GEIS 2013 guidelines for gastrointestinal sarcomas (GIST). Cancer Chemother Pharmacol 74(5):883–898. https://doi.org/10.1007/s00280-014-2547-0
Romano M, Giojelli A, Tamburrini O, Salvatore M (2003) Chemoembolization for hepatocellular carcinoma: effect of intraarterial lidocaine in peri- and post-procedural pain and hospitalization. La Radiol Med 105(4):350–355
Lee BM, Cata JP (2015) Impact of anesthesia on cancer recurrence. Revista Espanola de Anestesiologia y Reanimacion 62(10):570–575. https://doi.org/10.1016/j.redar.2015.04.003
Mao L, Lin S, Lin J (2013) The effects of anesthetics on tumor progression. Int J Physiol Pathophysiol Pharmacol 5(1):1–10
Chen WK, Miao CH (2013) The effect of anesthetic technique on survival in human cancers: a meta-analysis of retrospective and prospective studies. PLoS One 8(2):e56540. https://doi.org/10.1371/journal.pone.0056540
Zhang L, Hu R, Cheng Y, Wu X, Xi S, Sun Y, Jiang H (2017) Lidocaine inhibits the proliferation of lung cancer by regulating the expression of GOLT1A. Cell Prolif. https://doi.org/10.1111/cpr.12364
Xing W, Chen DT, Pan JH, Chen YH, Yan Y, Li Q, Xue RF, Yuan YF, Zeng WA (2017) Lidocaine induces apoptosis and suppresses tumor growth in human hepatocellular carcinoma cells in vitro and in a xenograft model in vivo. Anesthesiology 126(5):868–881. https://doi.org/10.1097/ALN.0000000000001528
Piegeler T, Schlapfer M, Dull RO, Schwartz DE, Borgeat A, Minshall RD, Beck-Schimmer B (2015) Clinically relevant concentrations of lidocaine and ropivacaine inhibit TNFalpha-induced invasion of lung adenocarcinoma cells in vitro by blocking the activation of Akt and focal adhesion kinase. Br J Anaesth 115(5):784–791. https://doi.org/10.1093/bja/aev341
Li K, Yang J, Han X (2014) Lidocaine sensitizes the cytotoxicity of cisplatin in breast cancer cells via up-regulation of RARbeta2 and RASSF1A demethylation. Int J Mol Sci 15(12):23519–23536. https://doi.org/10.3390/ijms151223519
Xuan W, Zhao H, Hankin J, Chen L, Yao S, Ma D (2016) Local anesthetic bupivacaine induced ovarian and prostate cancer apoptotic cell death and underlying mechanisms in vitro. Sci Rep 6:26277. https://doi.org/10.1038/srep26277
Bundscherer A, Malsy M, Gebhardt K, Metterlein T, Plank C, Wiese CH, Gruber M, Graf BM (2015) Effects of ropivacaine, bupivacaine and sufentanil in colon and pancreatic cancer cells in vitro. Pharmacol Res 95–96:126–131. https://doi.org/10.1016/j.phrs.2015.03.017
Suehiro J, Hamakubo T, Kodama T, Aird WC, Minami T (2010) Vascular endothelial growth factor activation of endothelial cells is mediated by early growth response-3. Blood 115(12):2520–2532. https://doi.org/10.1182/blood-2009-07-233478
Xiang W, Ke Z, Zhang Y, Cheng GH, Irwan ID, Sulochana KN, Potturi P, Wang Z, Yang H, Wang J, Zhuo L, Kini RM, Ge R (2011) Isthmin is a novel secreted angiogenesis inhibitor that inhibits tumour growth in mice. J Cell Mol Med 15(2):359–374. https://doi.org/10.1111/j.1582-4934.2009.00961.x
Madri JA, Pratt BM (1986) Endothelial cell-matrix interactions: in vitro models of angiogenesis. J Histochem Cytochem 34(1):85–91
Kaba A, Laurent SR, Detroz BJ, Sessler DI, Durieux ME, Lamy ML, Joris JL (2007) Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology 106(1):11–18 (discussion 15–16)
Davis GE, Senger DR (2005) Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circulation Res 97(11):1093–1107. https://doi.org/10.1161/01.RES.0000191547.64391.e3 pii]
Haraldsen G, Kvale D, Lien B, Farstad IN, Brandtzaeg P (1996) Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J Immunol 156(7):2558–2565
Ferrara N (2005) The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS 94:209–231
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol 7(5):359–371. https://doi.org/10.1038/nrm1911
Ronca R, Benkheil M, Mitola S, Struyf S, Liekens S (2017) Tumor angiogenesis revisited: Regulators and clinical implications. Med Res Rev. https://doi.org/10.1002/med.21452
Parums DV, Cordell JL, Micklem K, Heryet AR, Gatter KC, Mason DY (1990) JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol 43(9):752–757
Zhou CL, Li JJ, Ji P (2017) Propofol suppresses esophageal squamous cell carcinoma cell migration and invasion by down-regulation of sex-determining region Y-box 4 (SOX4). Med Sci Monit 23:419–427
Li H, Lu Y, Pang Y, Li M, Cheng X, Chen J (2017) Propofol enhances the cisplatin-induced apoptosis on cervical cancer cells via EGFR/JAK2/STAT3 pathway. Biomed Pharmacother 86:324–333. https://doi.org/10.1016/j.biopha.2016.12.036
Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP (2002) Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res 62(15):4491–4498
Le Gac G, Angenard G, Clement B, Laviolle B, Coulouarn C, Beloeil H (2017) Local anesthetics inhibit the growth of human hepatocellular carcinoma cells. Anesth Anal 125(5):1600–1609. https://doi.org/10.1213/ANE.0000000000002429
Piegeler T, Votta-Velis EG, Liu G, Place AT, Schwartz DE, Beck-Schimmer B, Minshall RD, Borgeat A (2012) Antimetastatic potential of amide-linked local anesthetics: inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology 117(3):548–559. https://doi.org/10.1097/ALN.0b013e3182661977
Auerbach R, Akhtar N, Lewis RL, Shinners BL (2000) Angiogenesis assays: problems and pitfalls. Cancer Metastasis Rev 19(1–2):167–172
Koodie L, Yuan H, Pumper JA, Yu H, Charboneau R, Ramkrishnan S, Roy S (2014) Morphine inhibits migration of tumor-infiltrating leukocytes and suppresses angiogenesis associated with tumor growth in mice. Am J Pathol 184(4):1073–1084. https://doi.org/10.1016/j.ajpath.2013.12.019
Guo XG, Wang S, Xu YB, Zhuang J (2015) Propofol suppresses invasion, angiogenesis and survival of EC-1 cells in vitro by regulation of S100A4 expression. Eur Rev Med Pharmacol Sci 19(24):4858–4865
Xu YB, Du QH, Zhang MY, Yun P, He CY (2013) Propofol suppresses proliferation, invasion and angiogenesis by down-regulating ERK-VEGF/MMP-9 signaling in Eca-109 esophageal squamous cell carcinoma cells. Eur Rev Med Pharmacol Sci 17(18):2486–2494
Hirata M, Sakaguchi M, Mochida C, Sotozono C, Kageyama K, Kuroda Y, Hirose M (2004) Lidocaine inhibits tyrosine kinase activity of the epidermal growth factor receptor and suppresses proliferation of corneal epithelial cells. Anesthesiology 100(5):1206–1210
Acknowledgements
This work was supported by a research grant provided by Hubei University of Arts and Science (RG2015668).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Research involving human participants and/or animals
All the procedures involving human participants were performed in accordance with the ethical standards of the national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All the procedures involving animals were conducted according to the guidelines approved by the Institutional Animal Care and Use Committee of Hubei University of Arts and Science
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, J., Hu, H. & Wang, X. Clinically relevant concentrations of lidocaine inhibit tumor angiogenesis through suppressing VEGF/VEGFR2 signaling. Cancer Chemother Pharmacol 83, 1007–1015 (2019). https://doi.org/10.1007/s00280-019-03815-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03815-4