Abstract
A basic developmental framework of the Larix leptolepis Gord male gametophyte is presented in detail by squashing technique. The duration of the meiosis stage was more than 6 months, and included a long diffuse stage during winter. This long duration of the diffuse appearance of the diplotene stage makes L. leptolepis a unique suitable experimental material for studying the structure and function of the diffuse stage of meiosis. In particular, the processes of desynapsing and unpairing, which so far have received little attention, can be examined in detail. In L. leptolepis, the chromosomes undergo a dramatic structural reorganization during the diffuse diplotene stage. Based on the clearly visible differences in chromosome morphology, the diffuse diplotene stage was divided into four periods with suggested nomenclature as follows: schizonema, pre-diffuse diplotene, diffuse diplotene and post-diffuse diplotene. Both simultaneous and successive microsporogenesis were observed within L. leptolepis, and there was no strict relationship between the microsporogenesis types and the tetrad configurations, which are strongly influenced by spindle orientation, especially during meiosis II. The mature pollen grain at pollination consists of five cells aligned in an axial row. The prothallial cells cannot be regarded as senescent cells because they remain capable of division.
Similar content being viewed by others
Introduction
The development of the male gametophyte in higher plants follows a tightly controlled sequence of events within the anther. Development can be divided into two major processes; microsporogenesis and microgametogenesis (Bedinger 1992). Microsporogenesis begins with the meiosis of microspore mother cells and ends with the formation of polarized haploid microspores. During this stage, meiosis bridges the transition from a diploid sporophyte to a haploid gametophyte generation. Microgametogenesis is initiated with the release of microspores from tetrads. Thereafter, the unicellular microspore develops into male gametophytes by one or several asymmetrical cell divisions (Barinova et al. 2002).
In general, the development of the male gametophyte of gymnosperms resembles that of angiosperms. However, microsporogenesis and microgametogenesis differ between them, especially in conifers (Andersson et al. 1969). First, there are the long duration of meiosis and the diffuse appearance of diplotene within most gymnosperm species as in conifers, which is typical for pollen mother cells (PMC) of Larix (Ekberg et al. 1968). Second, the male gametophyte of different gymnosperm species consists of a cell (the microspore) or a number of cells up to several score at dispersal (Singh 1978; Pacini et al. 1999), rather than bicellular or tricellular pollen.
Larix leptolepis is an economically important timber species, and there is vast literature on its male meiosis and gametophyte (Christiansen 1960; Chandler and Mavrodineanu 1965; Ekberg and Eriksson 1967; Eriksson 1968; Luomajoki 1977; Owens and Molder 1979; Romanova and Tret’yakova 2005). However, there is only fragmentary and scattered research on the complete development of the male gametophyte, especially the lengthy prophase, cytokinesis and microgametogenesis. Previous studies have demonstrated that meiosis in the pollen mother cell (PMC) of Larix extends from autumn to the end of winter or the beginning of the next spring (Christiansen 1960; Ekberg and Eriksson 1967). During autumn, male meiosis proceeds to the diplotene stage, and then has a resting stage after pachytene or early diplotene. During this stage, known as the diffuse stage, the cells undergoing meiosis remain in that phase for several months (Ekberg et al. 1968; Klášterská 1977). Although the significance of the diffuse stage has not yet been completely established, it is a striking step in meiotic prophase. To date, the occurrence of such a stage has been reported for all groups of plants; Angiospermae, Gymnospermae, Pteridophyta, Bryophyta, Fungi and Algae (Klášterská 1976), and, as first proposed by Wilson (1925), is likely a general feature of plant meiotic prophase (Moens 1964; Klášterská 1976, 1977). Although it is a widely reported phenomenon, important details in this process are still poorly understood. This is because there are few sufficiently detailed, continuous investigations of this stage and, in some cases, conclusions concerning chromosome morphology have been largely unfounded. Few published studies have clearly documented cytokinesis and illustrated the complete sequence of pollen development in L. leptolepis.
In this study, we aimed to extend the understanding of key meiotic events and processes with a special emphasis on the diffuse stage in L. leptolepis. We also aimed to demonstrate cytokinesis and callose deposition during sporogenesis, and establish a basic developmental framework of male gametophytes at the ordinal level.
Materials and methods
Male buds of L. leptolepis were collected from three 31–45-year-old trees growing in Dagujia of Liaoning Province, northeastern China. The development of the PMC was followed from late August 2006 to the beginning of May 2007, when mature pollen grains were present. To cover all the developmental stages from leptotene to mature pollen grain, generative buds were fixed each day, except from the diffuse stage to diakinesis during which generative buds were fixed only at interval of several weeks because of frost. Male buds were fixed in acetic alcohol (1:3) for 24 h and stored at −20°C until use.
Fixed male buds were rinsed briefly in water. The PMCs were dissected and squashed in carbol fuchsin to identify the meiotic stage of the bud. The sequence of the prophase stages and the transition from one stage to the other are followed by developmental time and by the mainly pre-established cytological criteria (Rhoades 1961; Whitehouse 1973). This approach makes it possible to analyze the correct sequence of meiotic prophase stages and the transition from one stage to the other, and gives detailed information about the timing of the stages.
When meiosis was in progress, some anthers were squashed in aniline blue [modified from Arens (1949) by the addition of 15% glycerol] to observe callosic wall formation by epifluorescence before the release of microspores. To observe pollen development, male buds were hydrolyzed in 1 N HCl at 60°C for 10 min, rinsed briefly in water, then mordanted in 4% iron alum solution overnight to make the interior structure of the pollen grains as visible as possible. After washing three times in water, male buds were stained in 1% hematoxylin for 3–4 h, and then squashed in 45% acetic acid.
Digital black-and-white images were recorded with a cooled charge-coupled device camera on the epifluorescence Olympus BX51 microscope and further improved for optimal brightness and contrast with Adobe Photoshop 7.0 image processing software.
Results
Microsporogenesis
The present study showed that the onset of meiosis in L. leptolepis occurred in early October, 2006. At this point, the nuclei of the microspore mother cells enlarged and entered into prophase of meiosis. Prophase of the first meiotic division in this species was long in duration due to a lengthy diffuse stage.
Prior to the onset of prophase I, PMCs, characterized by the marked large size and expansion of the nucleus, appeared roughly rectangular in outline and were closely packed. The nuclei of these cells were filled with dispersed chromatin, which stained faintly with carbol fuchsin. In addition, there was a single prominent nucleolus within each nucleus (Fig. 1a).
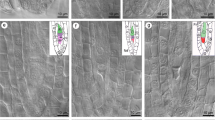
Stages of male meiosis from leptotene to pachytene in L. leptolepis. a PMCs appear roughly rectangular in outline and are closely packed. b Early leptotene; chromosomes first become visible as elongated strands. c Late leptotene; chromosomes are still completely unsynapsed but appear more condensed. d Early zygotene; chromatin is more condensed and the nuclei are polarized, suggesting initiation of the bouquet stage. e Late zygotene; major regions of homologs are closely associated and nuclei contain a noticeable interlocking. f Late zygotene; some regions begin to spiralize with homologs synapse. g Late zygotene; inversion or insertion/deletion ring and interlocking. h Early pachytene; bivalents still present a bouquet arrangement. i Late pachytene; telomeres are being released from the NE. j Late pachytene; enlargement of chromosomes shows clearly their double nature at this stage. The chromosomes had a granular structure. N nucleolus. Scale bar 15 μm
Leptotene is the first step in the condensation of DNA. At this stage, the nuclei of these cells were filled with thin thread-like chromatin. Small regions of the chromatin were thickened, indicating that the DNA was pronounced condensation. Overall, the total array of chromosomes appeared as a dense tangle of chromatin threads. Homologous chromosomes were still unpaired at this stage, and were difficult to be identified individually under the light microscope (Fig. 1b, c).
At the zygotene stage, the nuclei were clearly polarized. Chromosomes clustered at a small specific sector of the nuclear periphery in a compact configuration, suggesting that the bouquet stage had been initiated (Fig. 1d). The homologous chromosomes began to pair; Fig. 1d shows the homologs in close parallel association. Subsequently, paired homologs of each bivalent were intimately associated at many parts along their length, although they also showed some separated parts (Fig. 1e). Concomitant with chromosomes pairing, some parts of the bivalents started to spiralize (Fig. 1f). Certain nuclei contained noticeable interlocking, in which a pair of unsynapsed homologs was joined at the terminal regions, but in some interstitial fragments the two chromosomes completely encircled other chromosomes (Fig. 1e, g). We also discovered an inversion or insertion/deletion ring (Fig. 1g) at this stage, indicating that some changes occurred in the chromosome structure.
Pachytene nuclei were recognized by the thick fibers and the partial or complete dispersal of the bouquet. Generally, the nuclei were oval or round in shape. As homologous chromosomes were completely paired, the separated chromatids first lay side-by-side along their lengths (Fig. 1h, i). An enlarged image of this stage shows that these chromosomes were contracted and also shows their double nature (Fig. 1j). The chromosomes had a granular structure with a ‘pearl necklace’ appearance (Fig. 1j).
At early pachytene, chromosomes were still clustered at one side of the nucleus (Fig. 1h) indicating the persistence of the bouquet, up to the early pachytene stage. By the end of pachytene, however, the bouquet configuration disappeared and chromosome ends were displayed evenly around the inner surface of the nuclear envelope (Fig. 1i). In addition, the interlockings of bivalents, as described in the zygotene, were not observed in any pachytene nuclei observed, suggesting that interlocking was generally resolved prior to pachytene.
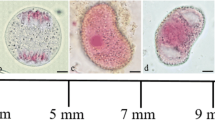
According to the classic meiosis framework, breakdown of synaptonemal complexes (SCs) marks the end of the pachytene stage and the beginning of diplotene. However, there was a transitional stage, a long diffuse stage, in L. leptolepis. This stage lasted approximately 5 months, generally from the end of October in the first year to late March of the second. During the diffuse stage, the chromosomes underwent dramatic structural reorganization. According to the morphological changes of chromosomes observed using light microscopy, the process of the diffuse stage was divided into four periods. First, the chromosomes passed through extreme decondensation and elongation with the gradual and progressive separation of the homologs at various points along their lengths. At the beginning of the diffuse diplotene, the closely paired homologs started decondensing rapidly, and separated longitudinally into two distinct threads. The two threads of the bivalent then became thinner and separated in some regions along the whole bivalent (Fig. 2a). Some parts of the chromosome despiralized more rapidly than others, which resulted in thin and thick parts alternating rather irregularly along the chromosome thread. The nucleus was visible as a network of such chromatin threads. The separated segments of bivalents, however, were still associated in parallel as at the zygotene stage, except at several contact points (Fig. 2a). Second, the chromosomes began to reorganize into a new specific configuration, where they were interconnected in a woolly multi-string-like structure (Fig. 2b–f). As the homologs separated further, the parallel-associated chromatin threads rapidly reorganized and became thicker than at the previous stage. Subsequently, “bubble-like” structures occurred along the whole bivalent due to variation in the degree of chromosome separation (Fig. 2b, c). After that, the chromosomes reorganized into a series of loops and clustered irregularly (Fig. 2d), and then showed an interconnected woolly multi-string-like appearance (Fig. 2e). Based on our observations, this period lasted about 2 weeks from early to mid November in 2006. In addition, this stage was characterized by a prominent large nucleolus (Fig. 2b–e). Third, the conventional diffuse stage was reached. In late November, the interconnected multi-string-like structure disappeared (Fig. 2g). The clustered chromosomes were released and appeared to become loose and diffuse. The nucleus then entered a diffuse stage. The chromosomes at this stage became more extended and tangled. Chromosomes became more diffuse and the nucleus displayed an irregular network of thicker and thinner chromatin fibers (Fig. 2h–k). The nucleus stained very faintly, but the nucleolus was still pronounced (Figs. 3h–k, 4d). This stage lasted for about 4 months from early November to late March of the following year (Figs. 2a, 3e). Fourth, the renewed contraction of the homologous chromosomes took place by the end of diffuse stage, giving rise to “lampbrush-like” chromosomes. After the diffuse stage, the chromosomes further contracted and hair-like extensions appeared perpendicular to the chromosome axis, giving the “lampbrush” appearance (Fig. 3f, g). These were quite pronounced and persisted for 1–2 weeks. This lampbrush appearance of Larix chromosomes has not been described previously. At this point, the bivalents were held together at several points along their lengths (Fig. 3h). The bivalents, as a consequence, had a looped appearance (Fig. 3h). The gradual change from the end of the diffuse stage to diplotene is shown in Fig. 3f–i.
Diffuse stage. a Schizonema; beginning of the diffuse stage, homologs begin to separate and decondense in some places. b–d Pre-diffuse stage; homologs continue to separate and decondense irregularly. “Bubble-like” structures occur (arrows). e Chromosomes reorganize in a series loops and cluster irregularly. f Chromosomes have an interconnected woolly multi-string-like appearance. g–k Diffuse stage; nucleus is filled with an irregular network of thicker and thinner chromatin fibers and becomes more diffuse. N nucleolus. Scale bar 15 μm
Diffuse stage. a–e Diffuse stage; bivalents still cannot be identified. f Postdiffuse stage; bivalents begin to reappear with hair-like extensions, and chromosomes display a “lampbrush” appearance. g Postdiffuse stage; bivalents are very irregularly contracted and are often interconnected. h Post-diffuse stage; bivalents are held together at several points along their length, taking on the appearance of a series of loops. i Diplotene stage; bivalents continue to contract, all hair-like extensions have already disappeared. Scale bar 15 μm
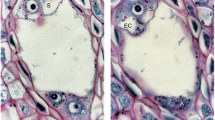
Stages of male meiosis from diakinesis to telophase II in L. leptolepis. a–l Carbol fuchsin staining. m–o Phase contrast microscopy images showing aniline blue staining. p Aniline blue staining. a Diakinesis; bivalents were irregularly dispersed. b Metaphase; fully condensed and aligned bivalents were presented on the equatorial plate. c Anaphase I; chromosomes move part toward the opposite poles. d Telophase I; chromosomes aggregate into compact masses at the poles. e Interkinesis. f Prophase II. g Metaphase II; spindle axes are oriented parallel to each other. h Metaphase II; spindle axes are oriented perpendicular to each other. i Anaphase II; perpendicular orientation of spindle axis is evident. j Anaphase II; parallel spindle axis. k Telophase II; four haploid nuclei are retained in a transitory syncytium in tetragon. l Telephase II; four haploid nuclei are retained in a transitory syncytium in tandem. m Organelle band appears in the equatorial zone. n Centrifugal cell plate of first cytoplasmic division. o–p Dyads after the first meiotic division. Callose surrounding the cells is visible in (p). Scale bar 15 μm
It is interesting to note that when the chromatin began to progressively recondense into distinct bivalents, the bivalents seemed first to be interconnected with hair-like extensions. Usually both interconnections and hair-like extensions disappeared from cells before diplotene, though they occasionally persisted in some cells (Fig. 3h, i).
By the end of the diplotene stage, the chromosomes quickly condensed to diakinesis. Homologous chromosomes were separated along their lengths and were joined only at chiasmata. All 12 bivalents were irregularly dispersed (Fig. 4a). At metaphase I, the nuclear envelope and nucleoli had disappeared and all pairs of homologous chromosomes were presented on the equatorial plate (Fig. 4b). During the next phase, anaphase I, the chiasmata were released and the chromosomes moved apart towards the opposite poles (Fig. 4c). However, chromatids of one chromosome segregated to the same pole. This stage was brief in duration. By the next stage, telophase I, the chromosomes aggregated into compact masses at the poles and temporarily decondensed (Fig. 4d). After a brief interphase (Fig. 4e), the nucleus entered meiosis II. The decondensed chromosomes recondensed and began migrating to the metaphase II plate (Fig. 4f). At metaphase II, the chromosomes lined up at the metaphase II plate, at the cell’s center (Fig. 4g, h). By the next stage, anaphase II, the sister chromatids separated and moved towards opposite cell poles (Fig. 4i, j). At the end of telophase II, there were four nuclei each with half of the original chromosome number (Fig. 4k, l). After cytokinesis, the four haploid microspores were produced from each PMC, which were arranged in various ways as illustrated in Fig. 5d–i. We found that tetrads were usually tetrahedral, tetragonal, or rhomboidal and were only rarely decussate, linear, or T-shaped (Fig. 5e–i). Variation in the orientations of the second meiotic axes affected the configuration of tetrads, giving rise to different shapes. For example, the spindle axes at meiosis II were occasionally oriented parallel to each other, resulting in the four haploid nuclei being retained in a transitory syncytium in a tetragon or in tandem (Fig. 4j–l).
Tetrads and the developmental sequence of pollen formation in L. leptolepis. a–i Images of stages stained with aniline blue. a Four cell walls developing centripetally towards the center of the tetrad. b Tetragonal tetrad with four centrifugal cell plates. c Tetrahedral tetrad with three intersporal walls visible. d Tetrahedral tetrad. e Tetragonal tetrad. f Decussate tetrad. g Rhomboidal tetrad. h T-shaped tetrad. i Linear tetrad. j–u Images of pollen stages stained with iron alum hematoxylin to show nuclei. j Uninucleate microspore. k Uninucleate microspores dividing. l Binucleate pollen grain after microspore mitosis with the first prothallial cell and the center cell. m Late binucleate pollen grain with the center cell in metaphase of mitosis. n Trinucleate pollen grain; center cell has just completed mitosis to form the second prothallial cell and the antheridial initial. o Late trinucleate pollen grain with dividing antheridial initial. p Four-celled stage of pollen grain; antheridial initial has divided to form antheridial cell (ac) adjacent to the second prothallial cell and the tube nucleus. r Antheridial cell rapidly increased in size for division. s Four-celled pollen grain with dividing antheridial cell. t Mature pollen grain showing two prothallial cells (pc), a stalk cell (sc), a body cell (bc), and nucleus of the tube cell (tc). q Binucleate prothallial cells (arrow). u One of three prothallial cells is in metaphase of mitosis (arrow). Scale bar 15 μm
By the end of the first meiosis, an organelle band (Fig. 4m) or a cell plate (Fig. 4n) appeared in the equatorial zone separating the daughter nuclei, indicating that both simultaneous and successive division occurred within Larix. During successive division, nuclei were delayed by a period of interphase during which a centrifugal cell plate formed, resulting in conspicuous dyads (Fig. 4o, p). During simultaneous division, the nuclei at this stage directly proceeded to meiosis II without forming a new nuclear membrane, and cytokinesis was achieved by the simultaneous formation of four to six centrifugal cell plates (Figs. 4m, 5a–c).
Microgametogenesis
Microspores separated by early April, after the callose walls had broken down. The haploid microspores soon became rounded and rapidly increased in size for the first division (Fig. 5j, k). This division resulted in two daughter cells; the prothallial cell and a cell known as the “center” cell (Singh 1978). The small prothallial cell was against the basal spore wall, and gradually became lenticular (Fig. 5l). The center cell continued to divide immediately (Fig. 5m), producing a second prothallial cell and a large cell, representing the antheridial initial (Fig. 5n). Soon after this, the antheridial initial divided into the tube nucleus and the antheridial cell (generative cell; Sterling 1963) (Fig. 5o, p). The antheridial cell then divided into the stalk cell and the body cell (Fig. 5r–t). These successive divisions resulted in five cells aligned in an axial row in the developing male gametophyte before pollination; the two prothallial cells, the stalk cell, the body cell, and the tube nucleus. No special cell wall was constructed around the tube nucleus. The wall of the developing pollen grain represents the wall of the tube cell as described in Douglas fir (Owens and Molder 1971). The cytoplasm of the pollen grain, therefore, is the cytoplasm of the large tube cell (Fig. 5t). During this process the haploid microspore divided four times, producing five-cell pollen. The pollen grain is shed at this stage.
It is interesting to note that certain prothallial cells divided again and produced often binucleate prothallial cells or multiple prothallial cells instead of becoming senescent (Fig. 5q, u).
Discussion
Although meiosis in L. leptolepis persists through the cold winter at the diffuse diplotene stage, this stage cannot be regarded as a resting stage as previously described in some literature (Ekberg and Eriksson 1967; Ekberg et al. 1968; Owens and Molder 1979). Our results clearly showed that during this stage the chromosomes undergo very profound changes in their organization. The large-scale reorganization of chromosomes allowed paired homologs to desynapse and unpair to exit prophase, which is necessary to reduce the diploid chromosome number by half.
From this point of view, an accurate description of chromosome morphology is critical to better understand the unpairing behavior of chromosomes in meiosis. Such observations can provide useful information on fundamental genetic events during this period. Moens (1964) gave the first detailed description of this stage in his study on Lycopersicon esculentum. He noticed that the homologs of a bivalent were separated after pachytene, and “schizonema” was proposed to describe the stage. Schizonema was followed by a diffuse stage and diplotene. Thereafter, pre-diffuse, diffuse, and post-diffuse diplotene were used to describe the morphological changes of chromosomes in postpachytene (Oud et al. 1979; Klášterská and Ramel 1979; Cardoso et al. 1986). For L. leptolepis, however, there is an additional period between pre-diffuse and diffuse diplotene during which the separated homologs of bivalents despiralize and reorganize into woolly multi-string-like structures (Fig. 2f). Based on our observations, the period between pachytene and diplotene in L. leptolepis can be divided into four stages: schizonema, pre-diffuse diplotene, mid-diffuse diplotene and post-diffuse diplotene. Schizonema is designated as the early diffuse stage, which is characterized by longitudinally separated homologous threads as described by Moens (1964). This stage has been described in male meiosis of many organisms (Moens 1964; Barry 1969; Nagl 1969; Sen 1969; Pogoslanz 1970; Klášterská and Natarajan 1974; Oud et al. 1979; Cawood and Jones 1980; Cardoso et al. 1986). Pre-diffuse diplotene is the transition from the early diplotene into the diffuse stage, during which the chromosomes despiralize and reorganize with the longitudinal separation of the bivalents. Mid-diffuse diplotene is characterized by a typical “diffuse” stage (Klášterská 1976; Seitz et al. 1996; Cattani and Papeschi 2004; Král et al. 2006). Post-diffuse diplotene is mainly characterized by recondensation of the homologous chromosomes to allow meiosis to enter the normal diplotene stage.
The significance of the diffuse diplotene stage is unclear, although the breakdown of SCs at the diffuse stage is a prerequisite for reformation of chromatid cores resulting from crossing over, i.e., chiasmata (Rufas et al. 1982; Stack 1991). Stack and Anderson (2001) proposed a model in, which chromosomes change from meiotic to mitotic organization at the diffuse stage corresponding to the active transcription of the decondensed chromosomes. Data from L. leptolepis were generally consistent with Stack and Anderson’s model. However, it is worth noting that male and female meiosis do not occur synchronously in L. leptolepis. Female meiosis, which is started and completed during spring, is delayed relative to male meiosis for about half a year. Therefore, the significance of the diffuse diplotene stage in L. leptolepis may be not only an adaptation to meiotic overwintering (Ekberg et al. 1968) under natural selection, but a regulation of synchrony among male and female gametes.
The microsporogenesis of Larix is a controversial topic (e.g., Saxton 1929; Christiansen 1960; Chandler and Mavrodineanu 1965; Rodkiewicz et al. 1984). Christiansen (1960) wrote: “…as far as is known, the wall formation of the four cells of a pollen tetrad of Larix normally takes place simultaneously…”, although he observed both simultaneous and successive microsporogenesis in Larix deciduas Mill. However, Rodkiewicz et al. (1984) claimed that successive cytokinesis occurs during microsporogenesis of Larix. Other authors (Saxton 1929; Chandler and Mavrodineanu 1965) maintained that cytokinesis may or may not occur following first division. The disagreement is mainly due to inadequate evidence. Our study showed that L. leptolepis had two types of cytokinesis, simultaneous and successive, with simultaneous being the predominant type (more than 90%). In the simultaneous type, the first meiotic division was followed directly by the second, and then cytokinesis and callose deposition occurred. The resulting tetrads were tetrahedral. In the successive type, a callose wall was laid down after the first meiotic division, forming a dyad. Tetrads resulting from successive division were tetragonal, T-shaped, linear, decussate or rhomboidal. However, simultaneous cytokinesis does not always produce tetrahedral tetrads because the configuration of the tetrad is strongly influenced by the orientation of meiotic spindles, especially during meiosis II. As a consequence, tetrad variation is remarkable in Larix; six different types were observed in this study, indicating some development and evolutionary versatility.
Although five-celled mature pollen has been reported in Larix (Owens and Molder 1979; Owens and Simpson 1986; Kosinski 1986; Said 1989), the complete course of pollen development has not been clearly shown due to the absence of sufficient detailed observations on pollen development. The present study showed that the pollen development proceeded as follows: first, the antheridial cell divided into the stalk cell and the body cell before shedding; second, the tube nucleus was produced by the third mitosis division and was situated at the side opposite to the prothallial cell; third, the mature pollen grain normally consisted of five cells resulting from four mitotic divisions.
It is often stated in the literature that conifers’ prothallial cells are usually senescent. However, the occasional proliferation of the prothallial cell in certain species of Pinaceae cannot be regarded as insignificant. Most researchers have concluded that the prothallial cell disintegrates on the basis of external characters and structure, but there have been no detailed studies on the internal structure. In the present research, we provide evidence that the prothallial cells, usually two cells in Larix (Sterling 1963; Singh 1978), continue to divide in some cases, forming di- or multi-nucleate cells (Fig. 5q, u). Prothallial cells with capacity for further cell division have also been described in several species in the Pinaceae, such as Picea excelsa (Miyake 1903; Pollock 1906) and Abies balsamea (Hutchinson 1914). This implies that prothallial cells may not be senescent and that their proliferation sometimes may be required to supplement the tube nucleus in the control and development of the pollen tubes. However, only future research can determine to what extent we are justified in suggesting that these phenomena are indicative of primary prothallial cells having a function instead of becoming senescent.
References
Andersson E, Ekberg I, Eriksson G (1969) A summary of meiosis investigations in conifers. Stud For Suec 70:1–20
Arens K (1949) Provo de calose por meio da microscopia a luz fluorescente e aplicacões do metodo. Lilloa 18:71–75
Barinova I, Zhexembekova M, Barsova E, Lukyanov S, Heberle-Bors E, Touraev A (2002) Antirrhinum majus microspore maturation and transient transformation in vitro. J Exp Bot 53:1119–1129
Barry EG (1969) The diffuse diplotene stage of meiotic prophase in Neurospora. Chromosma 26:119–129
Bedinger P (1992) The remarkable biology of pollen. Plant Cell 4:879–887
Cardoso H, Stoll M, Dutra A, Oliver G, Di Tomaso MV (1986) Characterization of the diffuse stage in the male meiotic prophase and karyotype of Scapteriscus borellii. Genetic 71:23–29
Cattani MV, Papeschi AG (2004) Nucleolus organizing regions and semi-persistent nucleolus during meiosis in Spartocera fusca (Thunberg) (Coreidae, Heteroptera). Hereditas 140:105–111
Cawood AH, Jones JK (1980) Chromosome behaviour during meiotic prophase in the Solanaceae. Chromosoma 80:57–68
Chandler C, Mavrodineanu S (1965) Meiosis in Larix laricina Koch Contr. Boyce Thompson Inst Pl Res 23:67–76
Christiansen H (1960) On the effect of low temperature on meiosis and pollen fertility in Larix deciduas Mill. Silvae Genet 9:72–78
Ekberg I, Eriksson G (1967) Development and fertility of pollen in three species of Larix. Hereditas 57:303–311
Ekberg I, Eriksson G, Šulíkov Á (1968) Meiosis and pollen formation in Larix. Hereditas 59:427–438
Eriksson G (1968) Temperature response of pollen mother cells in Larix and its importance for pollen formation. Stud For Suec 63:1–131
Hutchinson AH (1914) The male gametophyte of Abies balsamea. Bot Gaz 57:148–153
Klasterska I (1976) A new look on the role of the diffuse stage in problems of plant and animal meiosis. Hereditas 82:193–204
Klasterska I (1977) The concept of the prophase of meiosis. Hereditas 86:205–210
Klášterská I, Natarajan AT (1974) The role of the diffuse stage in the cytological problems of meiosis in Rosa. Hereditas 76:109–116
Klášterská I, Ramel C (1979) Prophase of plant meiosis: sequences and interpretation of stages. Genetica 51:15–20
Kosinski G (1986) Megagametogenesis, fertilization and embryo development in Larix deciduas. Can J For Res 16:1301–1309
Král J, Musilová J, Št’áhlavský F, Rězěǒ M, Akan Z, Edwards RL, Coyle FA, Almerje CR (2006) Evolution of the karyotype and sex chromosome systems in basal clades of araneomorph spiders (Araneae: Araneomorphase). Chromosome Res 14:859–880
Luomajoki A (1977) Effects of temperature on spermatophyte male meiosis. Hereditas 85:33–48
Miyake K (1903) On the development of the sexual organs and fertilization in Picea excelsa. Ann Bot 17:351–372
Moens PB (1964) A new interpretation of meiotic prophase in Lycopersicum esculentum (tomato). Chromosoma 15:231–242
Nagl W (1969) the course of the first meiotic prophase in Beta procumbens and in the F1 between B. vulgaris and B. procumbens. Theor Appl Genet 39:356–360
Oud JL, de Jong JH, de Rooij DG (1979) A sequential analysis of meiosis in the male mouse using a restricted spermatocyte population obtained by a hydroxyurea/triaziquone treatment. Chromosoma 71:237–248
Owens JN, Molder M (1971) Pollen development in Douglas fir (Pseudotsuga menziesii). Can J Bot 49:1263–1266
Owens JN, Molder M (1979) Sexual reproduction of Larix occidentalis. Can J Bot 57:2673–2690
Owens JN, Simpson S (1986) Pollen from conifers native to British Columbia. Can J For Res 16:955–967
Pacini E, Franchi GG, Ripaccioli M (1999) Ripe pollen structure and histochemistry of some gymnosperms. Plant Syst Evol 75:183–196
Pogoslanz HE (1970) Meiosis in the Djungarian hamster I. General pattern of male meiosis. Chromosoma 31:392–403
Pollock JB (1906) Variations in the pollen grain of Picea excelsa. Am Nat 49:253–286
Rhoades MM (1961) Meiosis. In: Brachet J, Mirsky AE (eds) The cell. Academic Press, New York, pp 1–75
Rodkiewicz B, Kudlicka K, Stobiecka H (1984) Patterns of amyloplast distribution during microsporogenesis in Tradescantia, Impatins and Larix. Acta Soc Bot Pol 53:437–441
Romanova LI, Tret’yakova IN (2005) Specific features of microsporogenesis in the Siberian larch growing under the conditions of technogenic load. Russ J Dev Biol 36:99–104
Rufas JS, Gime¨ nez-Martin G, Esponda P (1982) Presence of a chromatid core in mitotic and meiotic chromosomes of grasshoppers. Cell Biol Int Rep 6:261–267
Said C (1989) Some characteristics of pollen wall cytochemistry and ultrastructure in Japanese larch (Larix leptolepis Gord). Sex Plant Reprod 2:77–84
Saxton WT (1929) Notes on conifers. II. Some points in the morphology of Larix europaea D.C. Ann Bot 43:609–613
Seitz LC, Tang K, Cummings WJ, Zolan ME (1996) The rad9 gene of Coprinus cinereus encodes a proline-rich protein required for meiotic chromosome condensation and synapsis. Genetics 142:1105–1117
Sen SK (1969) Chromatin-organization during and after synapsis in cultured microsporocytes of Lilium in presence of mitomycin C and cyclohexinide. Exp Cell Res 55:123–127
Singh H (1978) Embryology of gymnosperms. Gebruder Borntraeger, Berlin
Stack SM (1991) Staining plant cells with silver. II. Chromosome cores. Genome 34:900–908
Stack SM, Anderson LK (2001) A model for chromosome structure during the mitotic and meiotic cell cycles. Chromosome Res 9:175–198
Sterling C (1963) Structure of the male gametophyte in gymnosperms. Biol Rev 38:167–203
Whitehouse HLK (1973) Towards an understanding of the mechanism of heredity. Arnold, London
Wilson EB (1925) The cell in development and heredity. The Macmillan Company, New York
Acknowledgments
We are grateful to Guang-Yuan Rao and Yan-Ping Guo for their valuable comments on the manuscript. This work was supported by the National High Technology Research and Development Program of China (Grant Nos. 2006AA100109, 2007AA021403, 2007AA10Z182 & 2008AA10Z126), the National Natural Science Foundation of China (Grant No. 30571517), the National “948” Program (N0. 2007-4-03).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Altman.
S.-G. Zhang and W.-H. Yang have contributed equally to this work.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Zhang, SG., Yang, WH., Qi, YC. et al. Development of male gametophyte of Larix leptolepis Gord. with emphasis on diffuse stage of meiosis. Plant Cell Rep 27, 1687–1696 (2008). https://doi.org/10.1007/s00299-008-0579-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-008-0579-9