Abstract
Objective
To investigate multi-echo chemical shift-encoded MRI-based mapping of proton density fat fraction (PDFF) and fat-corrected R2* in bone marrow as biomarkers for osteoporosis assessment.
Methods
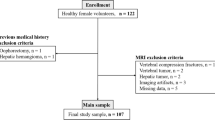
Fifty-one patients (28 female; mean age 69.7 ± 9.0 years) underwent dual energy X-ray absorptiometry (DXA). On the basis of the t score, 173 valid vertebrae bodies were divided into three groups (healthy, osteopenic and osteoporotic). Three echo chemical shift-encoded MRI sequences were acquired at 3 T. PDFF and R2* with correction for multiple-peak fat (R2*MP) were measured for each vertebral body. Kruskal–Wallis test and post hoc analysis were performed to evaluate differences between groups. Further, the area under the curve (AUC) for each technique was calculated using logistic regression analysis.
Results
On the basis of DXA, 92 samples were normal (53 %), 47 osteopenic (27 %) and 34 osteoporotic (20 %). PDFF was increased in osteoporosis compared with healthy (P = 0.007). R2*MP showed significant differences between normal and osteopenia (P = 0.004), and between normal and osteoporosis (P < 0.001). AUC to differentiate between normal and osteoporosis was 0.698 for R2*MP, 0.656 for PDFF and 0.74 for both combined.
Conclusion
PDFF and R2*MP are moderate biomarkers for osteoporosis. PDFF and R2*MP combination might improve the prediction in differentiating healthy subjects from those with osteoporosis.
Key Points
• Magnetic resonance imaging offers new insights into bone composition.
• PDFF and R2* are moderate biomarkers for the assessment of osteoporosis.
• R2* mapping for staging of osteoporosis is affected by bone marrow fat.
• Multi-peak spectral modelling improved the relationship between osteoporosis and R2*.
• PDFF/multi-peak fat-corrected R2* together might improve differentiation between healthy and osteoporotic subjects.




Similar content being viewed by others
References
WHO Study Group (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 843:1–129
Glaser DL, Kaplan FS (1997) Osteoporosis. Definition and clinical presentation. Spine 22:12S–16S
McDonnell JM, Lane JM, Zimmerman PA (1987) Osteoporosis: definition, risk factors, etiology, and diagnosis. AAOHN J 35:527–530
Sanfélix-Gimeno G, Sanfelix-Genovés J, Hurtado I et al (2013) Vertebral fracture risk factors in postmenopausal women over 50 in Valencia, Spain. A population-based cross-sectional study. Bone 52:393–399
Blake GM, Fogelman I (2007) The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J 83:509–517
Wehrli FW, Ford JC, Haddad JG (1995) Osteoporosis: clinical assessment with quantitative MR imaging in diagnosis. Radiology 196:631–641
Damilakis J, Maris T, Papadokostakis G et al (2004) Discriminatory ability of magnetic resonance T2* measurements in a sample of postmenopausal women with low-energy fractures: a comparison with phalangeal speed of sound and dual x-ray absorptiometry. Invest Radiol 39:706–712
Maris TG, Damilakis J, Sideri L et al (2004) Assessment of the skeletal status by MR relaxometry techniques of the lumbar spine: comparison with dual X-ray absorptiometry. Eur J Radiol 50:245–256
Sirlin CB, Reeder SB (2010) Magnetic resonance imaging quantification of liver iron. Magn Reson Imaging Clin N Am 18:359–381, ix
Kuehn J-P, Hernando D, Muñoz Del Rio A et al (2012) Effect of multipeak spectral modeling of fat for liver iron and fat quantification: correlation of biopsy with MR Imaging results. Radiology 265:133–142
Hernando D, Kuehn J-P, Mensel B et al (2012) R2* estimation using “in-phase” echoes in the presence of fat: the effects of complex spectrum of fat. J Magn Reson Imaging. doi:10.1002/jmri.23851
Reeder SB, Robson PM, Yu H et al (2009) Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging 29:1332–1339
Baum T, Yap SP, Karampinos DC et al (2012) Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging 35:117–124
Griffith JF, Yeung DKW, Antonio GE et al (2006) Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology 241:831–838
Li X, Kuo D, Schafer AL et al (2011) Quantification of vertebral bone marrow fat content using 3 Tesla MR spectroscopy: reproducibility, vertebral variation, and applications in osteoporosis. J Magn Reson Imaging 33:974–979
Yeung DKW, Griffith JF, Antonio GE et al (2005) Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging 22:279–285
Griffith JF, Yeung DKW, Antonio GE et al (2005) Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology 236:945–951
Tang GY, Lv ZW, Tang RB et al (2010) Evaluation of MR spectroscopy and diffusion-weighted MRI in detecting bone marrow changes in postmenopausal women with osteoporosis. Clin Radiol 65:377–381
Reeder SB, Hu HH, Sirlin CB (2012) Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging 36:1011–1014
Meisamy S, Hines CDG, Hamilton G et al (2011) Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 258:767–775
Yokoo T, Shiehmorteza M, Hamilton G et al (2011) Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology 258:749–759
Kang GH, Cruite I, Shiehmorteza M et al (2011) Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging 34:928–934
Hamilton G, Yokoo T, Bydder M et al (2011) In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed 24:784–790
Yu H, Shimakawa A, McKenzie CA et al (2008) Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 60:1122–1134
Hernando D, Liang Z-P, Kellman P (2010) Chemical shift-based water/fat separation: a comparison of signal models. Magn Reson Med 64:811–822
de Bazelaire CMJ, Duhamel GD, Rofsky NM, Alsop DC (2004) MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 230:652–659
Hines CDG, Frydrychowicz A, Hamilton G et al (2011) T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging 33:873–881
Reeder SB, Sirlin CB (2010) Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am 18:337–357, ix
Gokalp G, Mutlu FS, Yazici Z, Yildirim N (2011) Evaluation of vertebral bone marrow fat content by chemical-shift MRI in osteoporosis. Skeletal Radiol 40:577–585
Wehrli FW, Ma J, Hopkins JA, Song HK (1998) Measurement of R′2 in the presence of multiple spectral components using reference spectrum deconvolution. J Magn Reson 131:61–68
Rosenthal H, Thulborn KR, Rosenthal DI et al (1990) Magnetic susceptibility effects of trabecular bone on magnetic resonance imaging of bone marrow. Invest Radiol 25:173–178
Funke M, Bruhn H, Vosshenrich R et al (1994) The determination of the T2* relaxation time for characterizing trabecular bone. Rofo 161:58–63
Reeder SB, Brittain JH, Grist TM, Yen Y-F (2007) Least-squares chemical shift separation for (13)C metabolic imaging. J Magn Reson Imaging 26:1145–1152
Bolotin HH, Sievänen H (2001) Inaccuracies inherent in dual-energy X-ray absorptiometry in vivo bone mineral density can seriously mislead diagnostic/prognostic interpretations of patient-specific bone fragility. J Bone Miner Res 16:799–805
Acknowledgments
This work is part of the research project Greifswald Approach to Individualized Medicine (GANI_MED). The GANI_MED consortium is funded by the German Federal Ministry of Education and Research (FKZ 03IS2061A) and the Ministry of Cultural Affairs of the Federal State of Mecklenburg West Pomerania (UG 09 033). We also acknowledge the support of the National Institutes of Health (NIH) (R01 DK083380 and R01 DK088925).
We acknowledge the help of the medical technical assistants of the Department of Radiology and Neuroradiology of the University Hospital Greifswald.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kühn, JP., Hernando, D., Meffert, P.J. et al. Proton-density fat fraction and simultaneous R2* estimation as an MRI tool for assessment of osteoporosis. Eur Radiol 23, 3432–3439 (2013). https://doi.org/10.1007/s00330-013-2950-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-2950-7