Abstract
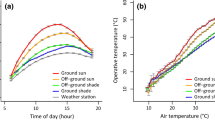
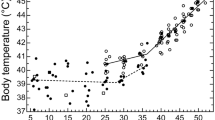
Nightjars represent a model taxon for investigating physiological limits of heat tolerance because of their habit of roosting and nesting in sunlit sites during the heat of the day. We investigated the physiological responses of Rufous-cheeked nightjars (Caprimulgus rufigena) and Freckled nightjars (Caprimulgus tristigma) to high air temperatures (T a) by measuring body temperature (T b), resting metabolic rate (RMR) and total evaporative water loss (TEWL) at T a ranging from 10 to 56 °C. Both species became hyperthermic at T a > T b. Lower critical limits of thermoneutrality occurred at T a between 35 and 37 °C, whereas we detected no clear upper critical limits of thermoneutrality. Between T a ≈ 37.0 and 39.9 °C, rates of TEWL increased rapidly with T a. At T a ≥ 40 °C, fractional increases in mass-specific TEWL rates were 78–106% of allometric predictions. Increasing evaporative heat dissipation incurred only small metabolic costs, with the RMR of neither species ever increasing by more than 20% above thermoneutral values. Consequently, both species displayed extremely efficient evaporative cooling; maximum evaporative heat dissipation was equivalent to 515% of metabolic heat production (MHP) at T a ≈ 56 °C in C. rufigena and 452% of MHP at T a ≈ 52 °C in C. tristigma. Our data reiterate that caprimulgids have evolved an efficient mechanism of evaporative cooling via gular fluttering, which minimizes metabolic heat production at high T a and reduces total heat loads. This likely aids in reducing TEWL rates and helps nightjars cope with some of the most thermally challenging conditions experienced by any bird.




Similar content being viewed by others
Abbreviations
- EHL:
-
Evaporative heat loss
- M b :
-
Body mass
- MHP:
-
Metabolic heat production
- RMR:
-
Resting metabolic rate
- T a :
-
Air temperature
- T b :
-
Body temperature
- T e :
-
Operative temperature
- TEWL:
-
Total evaporative water loss
- T lc :
-
Lower critical limit of thermoneutrality
- T uc :
-
Upper critical limit of thermoneutrality
References
Amat JA, Masero JA (2004) Predation risk on incubating adults constrains the choice of thermally favourable nest sites in a plover. Anim Behav 67:293–300
Arad Z, Gavrieli-Levin I, Eylath U, Marder J (1987) Effect of dehydration on cutaneous water evaporation in heat-exposed pigeons (Columba livia). Physiol Zool 60:623–630
Bartholomew GA, Cade TJ (1963) The water economy of land birds. Auk 80:504–539
Bartholomew GA, Dawson WR (1979) Thermoregulatory behavior during incubation in heermann’s gulls. Physiol Zool 52:422–437
Bartholomew GA, Hudson JW, Howell TR (1962) Body temperature, oxygen consumption, evaporative water loss and heart rate in the poor-will. Condor 64:117–125
Bartholomew GA, Lasiewski RC, Crawford EC Jr (1968) Patterns of panting and gular flutter in cormorants, pelicans, owls, and doves. Condor 70:31–34
Bartoń K (2015) MuMIn: multi-model inference. R package version 1.15.1. http://CRAN.R-project.org/package=MuMIn
Bates D (2006) [R] lmer, p-values and all that. https://stat.ethz.ch/pipermail/r-help/2006-May/094765. Accessed 10 July 2015
Bates D, Maechler M, Bolker B, Walker S (2015) lme4: linear mixed-effects models using eigen and S4. R package version 1.1-9. https://CRAN.R-project.org/package=lme4
Boyles JG, Smit B, McKechnie AE (2012) Variation in body temperature is related to ambient temperature but not experimental manipulation of insulation in two small endotherms with different thermoregulatory patterns. J Zool 287:224–232
Brigham RM, McKechnie AE, Doucette LI, Geiser F (2012) Heterothermy in caprimulgid birds: a review of inter- and intraspecific variation in free-ranging populations. In: Ruf T, Bieber C, Arnold W, Millesi E (eds) Living in a seasonal world: thermoregulatory and metabolic adaptation. Springer, Berlin, Heidelberg, pp 175–187
Calder WA Jr, Schmidt-Nielsen K (1966) Evaporative cooling and respiratory alkalosis in the pigeon. Proc Natl Acad Sci USA 55:750–756
Chaui-Berlinck JG, Monteiro LHA, Navas CA, Bicudo JEPW (2002) Temperature effects on energy metabolism: a dynamic system analysis. Proc Roy Soc Lond B Biol 269:15–19
Chaui-Berlinck JG, Navas CA, Monteiro LHA, Bicudo JEPW (2004) Temperature effects on a whole metabolic reaction cannot be inferred from its components. Proc Roy Soc Lond B Biol 271:1415–1419
Cleere N, Nurney D (1998) Nightjars: a guide to the nightjars, nighthawks, and their relatives. Yale University Press, New Haven
Core Team R (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Cowles RB, Dawson WR (1951) A cooling mechanism of the Texas nighthawk. Condor 53:19–22
Crawley MJ (2007) The R book. Wiley, West Sussex
Cunningham SJ, Martin RO, Hojem CL, Hockey PAR (2013) Temperatures in excess of critical thresholds threaten nestling growth and survival in a rapidly-warming arid savanna: a study of common fiscals. PLoS One 8(9):e74613
Dawson WR (1958) Relation of oxygen consumption and evaporative water loss to temperature in the cardinal. Physiol Zool 31:37–48
Dawson WR (1982) Evaporative losses of water by birds. Comp Biochem Phys A 71:495–509
Dawson WR, Fisher CD (1969) Responses to temperature by the spotted nightjar (Eurostopodus guttatus). Condor 71:49–53
Dexter RW (1956) Further banding and nesting studies of the eastern nighthawk. Bird-Banding 27:9–16
Doucette LI, Geiser F (2008) Seasonal variation in thermal energetics of the Australian owlet-nightjar (Aegotheles cristatus). Comp Biochem Phys A 151:615–620
du Plessis KL, Martin RO, Hockey PAR, Cunningham SJ, Ridley AR (2012) The costs of keeping cool in a warming world: implications of high temperatures for foraging, thermoregulation and body condition of an arid-zone bird. Glob Change Biol 18:3063–3070
du Prel JB, Hommel G, Röhrig B, Blettner M (2009) Confidence interval or p-value? Dtsch Arztebl Int 106:335–339
Faraway JJ (2006) Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. Chapman & Hall/CRC, Boca Raton
Ganey JL (2004) Thermal regimes of Mexican Spotted Owl nest stands. Southwest Nat 49:478–486
Grant GS (1982) Avian incubation: egg temperature, nest humidity, and behavioral thermoregulation in a hot environment. Ornithol Monogr 30:1–75
Haugen MJ, Tieleman BI, Williams JB (2003) Phenotypic flexibility in cutaneous water loss and lipids of the stratum corneum. J Exp Biol 206:3581–3588
Heldmaier G, Ruf T (1992) Body temperature and metabolic rate during natural hypothermia in endotherms. J Comp Physiol B 162:696–706
Ingels J, Ribot JH, de Jong BHJ (1984) Vulnerability of eggs and young of the blackish nightjar (Caprimulgus nigrescens) in Suriname. Auk 101:388–391
Koller M (2015) robustlmm: robust linear mixed effects models. R package version 1.7-6. http://CRAN.R-project.org/package=robustlmm
Lane JE, Swanson DL, Brigham RM, McKechnie AE (2004) Physiological responses to temperature by whip-poor-wills: more evidence for the evolution of low metabolic rates in caprimulgiformes. Condor 106:921–925
Lasiewski RC (1969) Physiological responses to heat stress in the poorwill. Am J Physiol 217:1504–1509
Lasiewski RC, Bartholomew GA (1966) Evaporative cooling in the Poor-will and the Tawny Frogmouth. Condor 68:253–262
Lasiewski RC, Dawson WR (1964) Physiological responses to temperature in the common nighthawk. Condor 66:477–490
Lasiewski RC, Seymour RS (1972) Thermoregulatory responses to heat stress in four species of birds weighing approximately 40 grams. Physiol Zool 45:106–118
Lasiewski RC, Acosta AL, Bernstein MH (1966) Evaporative water loss in birds-I. Characteristics of the open flow method of determination, and their relation to estimates of thermoregulatory ability. Comp Biochem Physiol 19:445–457
Lasiewski RC, Bernstein MH, Ohmart RD (1971) Cutaneous water loss in the roadrunner and poor-will. Condor 73:470–472
Lighton JRB (2008) Measuring metabolic rates: a manual for scientists. Oxford University Press, Oxford
Ligon JD (1968) The biology of the Elf Owl, (Micrathene whitneyi). Miscellaneous Publications Museum of Zoology, University of Michigan No. 136
Ligon JD (1969) Some aspects of temperature relations in small owls. Auk 86:458–472
Lovegrove BG, Canale C, Levesque D, Fluch G, Řeháková-Petrů M, Ruf T (2014) Are tropical small mammals physiologically vulnerable to arrhenius effects and climate change? Physiol Biochem Zool 87:30–45. doi:10.1086/673313
Marder J, Arieli Y (1988) Heat balance of acclimated pigeons (Columba livia) exposed to temperatures up to 60 °C Ta. Comp Biochem Physiol A 91:165–170
Marder J, Withers PC, Philpot RG (2003) Patterns of cutaneous water evaporation by Australian pigeons. Isr J Zool 49:111–129
McKechnie AE, Wolf BO (2004) Partitioning of evaporative water loss in white-winged doves: plasticity in response to short-term thermal acclimation. J Exp Biol 207:203–210
McKechnie AE, Wolf BO (2010) Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol Lett 6:253–256
McKechnie AE, Noakes MJ, Smit B (2015) Global patterns of seasonal acclimatization in avian resting metabolic rates. J Ornithol 156:367–376. doi:10.1007/s10336-015-1186-5
McKechnie AE, Smit B, Whitfield MC, Noakes MJ, Talbot WA, Garcia M, Gerson AR, Wolf BO (2016a) Avian thermoregulation in the heat: evaporative cooling capacity in an archetypal desert specialist, Burchell’s sandgrouse (Pterocles burchelli). J Exp Biol 219:2137–2144
McKechnie AE, Whitfield MC, Smit B, Gerson AR, Smith EK, Talbot WA, McWhorter TJ, Wolf BO (2016b) Avian thermoregulation in the heat: efficient evaporative cooling allows for extreme heat tolerance in four southern Hemisphere columbids. J Exp Biol 219:2145–2155
McNab BK (2006) The relationship among flow rate, chamber volume and calculated rate of metabolism in vertebrate respirometry. Comp Biochem Phys A 145:287–294
Muñoz-Garcia A, Cox RM, Williams JB (2008) Phenotypic flexibility in cutaneous water loss and lipids of the stratum corneum in house sparrows (Passer domesticus) following acclimation to high and low humidity. Physiol Biochem Zool 81:87–96
Noakes MJ, Wolf BO, McKechnie AE (2016) Seasonal and geographical variation in heat tolerance and evaporative cooling capacity in a passerine bird. J Exp Biol. doi:10.1242/jeb.132001
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2015) nlme: linear and nonlinear mixed effects models. R package version 3.1-121. http://CRAN.R-project.org/package=nlme
Porter WP, Gates DM (1969) Thermodynamic equilibria of animals with environment. Ecol Monogr 39:227–244
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Smit B, McKechnie AE (2010) Avian seasonal metabolic variation in a subtropical desert: basal metabolic rates are lower in winter than in summer. Funct Ecol 24:330–339
Smit B, Boyles JG, Brigham RM, McKechnie AE (2011) Torpor in dark times: patterns of heterothermy are associated with the lunar cycle in a nocturnal bird. J Biol Rhythm 26:241–248
Smith EK, O’Neill J, Gerson AR, Wolf BO (2015) Avian thermoregulation in the heat: resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert doves and quail. J Exp Biol 218:3636–3646
Snyder GK, Nestler JR (1990) Relationships between body temperature, thermal conductance, Q10 and energy metabolism during daily torpor and hibernation in rodents. J Comp Physiol B 159:667–675
Sonderegger D (2012) SiZer: significant zero crossings. R package version 0.1-4. http://CRAN.R-project.org/package=SiZer
Spottiswoode CN, Jackson HD (2005a) Rufous-cheeked nightjar. In: Hockey PAR, Dean WRJ, Ryan PG (eds) Roberts birds of southern Africa, 7th edn. The Trustees of the John Voelcker Bird Book Fund, Cape Town, pp 271–272
Spottiswoode CN, Jackson HD (2005b) Freckled nightjar. In: Hockey PAR, Dean WRJ, Ryan PG (eds) Roberts birds of southern Africa, 7th edn. The Trustees of the John Voelcker Bird Book Fund, Cape Town, pp 266–268
Steyn P (1971) Notes on the breeding biology of the freckled nightjar. Ostrich 42:179–188
Swanson DL, Drymalski MW, Brown JR (1996) Sliding vs static cold exposure and the measurement of summit metabolism in birds. J Therm Biol 21:221–226
Tattersall GJ, Sinclair BJ, Withers PC, Fields PA, Seebacher F, Cooper CE, Maloney SK (2012) Coping with thermal challenges: physiological adaptations to environmental temperatures. Compr Physiol 2:2151–2202
Tieleman BI, Williams JB (1999) The role of hyperthermia in the water economy of desert birds. Physiol Biochem Zool 72:87–100
Tieleman BI, Williams JB, Buschur ME (2002a) Physiological adjustments to arid and mesic environments in larks (Alaudidae). Physiol Biochem Zool 75:305–313
Tieleman BI, Williams JB, LaCroix F, Paillat P (2002b) Physiological responses of houbara bustards to high ambient temperatures. J Exp Biol 205:503–511
Trost CH (1972) Adaptations of horned larks (Eremophila alpestris) to hot environments. Auk 89:506–527
Walsberg GE, Voss-Roberts KA (1983) Incubation in desert-nesting doves: mechanisms for egg cooling. Physiol Zool 56:88–93
Weathers WW (1981) Physiological thermoregulation in heat-stressed birds: consequences of body size. Physiol Zool 54:345–361
Weathers WW (1997) Energetics and thermoregulation by small passerines of the humid, lowland tropics. Auk 114:341–353
Weathers WW, Caccamise DF (1978) Seasonal acclimatization to temperature in monk parakeets. Oecologia 35:173–183
Weathers WW, Schoenbaechler DC (1976) Regulation of body temperature in the budgerygah, Melopsittacus undulatus. Aust J Zool 24:39–47
Weller MW (1958) Observations on the incubation behavior of a common nighthawk. Auk 75:48–59
Whitfield MC, Smit B, McKechnie AE, Wolf BO (2015) Avian thermoregulation in the heat: scaling of heat tolerance and evaporative cooling capacity in three southern African arid-zone passerines. J Exp Biol 218:1705–1714
Whittow GC (1976) Regulation of body temperature. In: Sturkie PD (ed) Avian physiology, 3rd edn. Springer, New York, pp 146–173
Williams JB (1996) A phylogenetic perspective of evaporative water loss in birds. Auk 113:457–472
Williams JB (1999) Heat production and evaporative water loss of dune larks from the Namib desert. Condor 101:432–438
Williams JB, Tieleman BI (2005) Physiological adaptation in desert birds. Bioscience 55:416–425
Williams JB, Muñoz-Garcia A, Champagne A (2012) Climate change and cutaneous water loss of birds. J Exp Biol 215:1053–1060
Withers PC (1977) Respiration, metabolism, and heat exchange of euthermic and torpid poorwills and hummingbirds. Physiol Zool 50:43–52
Withers PC (1992) Comparative animal physiology. Saunders College Publishing, Orlando
Withers PC, Williams JB (1990) Metabolic and respiratory physiology of an arid-adapted Australian bird, the spinifex pigeon. Condor 92:961–969
Wolf BO, Walsberg GE (1996) Respiratory and cutaneous evaporative water loss at high environmental temperatures in a small bird. J Exp Biol 199:451–457
Acknowledgements
We thank Duncan MacFadyen and E. Oppenheimer & Son for allowing us to conduct research on their properties, Pieter and Verencia Benade for their hospitality and access to their farm. Cathy Bester, Ryno Kemp and Pieter Erasmus provided valuable assistance in the field. Lastly, we thank Bruce Woodroffe and Awesome Tools (Cape Town, South Africa) for discounted lighting equipment. This paper was improved through the helpful comments of two anonymous reviewers. Funding was provided by the DST-NRF Center of Excellence at the Percy FitzPatrick Institute and University of Pretoria. This material is based on work supported by the National Science Foundation under IOS-1122228 to B.O.W. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This study was approved by the University of Pretoria Animal Ethics Committee (Project EC068-13) and complies with current South African laws.
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
O’Connor, R.S., Wolf, B.O., Brigham, R.M. et al. Avian thermoregulation in the heat: efficient evaporative cooling in two southern African nightjars. J Comp Physiol B 187, 477–491 (2017). https://doi.org/10.1007/s00360-016-1047-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-1047-4