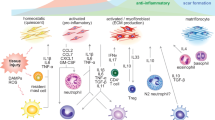
Abstract
The heart contains various types of cells, including cardiomyocytes, cardiac fibroblasts, many kinds of immune cells and vascular cells. Initial studies mainly focused on cardiomyocytes, which directly reflect the contractile function of the heart. Recently, pivotal functions of cardiac fibroblasts have been revealed in the maintenance of cardiac function, physiological cardiac remodeling after heart stress and pathological remodeling using genetically engineered mouse models, like the fibroblast-specific gene knockout mouse, bone marrow transplantation and immune cell-specific gene knockout. Moreover, chronic inflammation is considered to be a basic pathological mechanism that underlies various diseases, including heart failure. In the development of heart failure, the contributions of immune cells like T lymphocytes and monocyte/macrophage lineage cells have been also reported. Immune cells have diverse and multiple functions in regulating both pro-inflammatory effects and the resolution of heart failure. On the one hand, immune cells have protective effects to compensate for and overcome heart stresses. On the other hand, they also contribute to sustained inflammation and result in the development of heart failure. These observations prompted a shift in the heart-related studies to include the complex communications between cardiomyocytes and other kinds of cardiac cells, including inflammatory cells residing in or recruited to the heart. This review will summarize the current knowledge regarding cell–cell interactions during cardiac remodeling and the development of heart failure. We will especially focus on the interactions among cardiomyocytes, cardiac fibroblasts and immune cells.



Similar content being viewed by others
References
Accornero F, van Berlo JH, Benard MJ, Lorenz JN, Carmeliet P, Molkentin JD (2011) Placental growth factor regulates cardiac adaptation and hypertrophy through a paracrine mechanism. Circ Res 109:272–280. doi:10.1161/CIRCRESAHA.111.240820
Adam O, Lohfelm B, Thum T, Gupta SK, Puhl SL, Schafers HJ, Bohm M, Laufs U (2012) Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res Cardiol 107:278. doi:10.1007/s00395-012-0278-0
Afzali B, Lombardi G, Lechler RI, Lord GM (2007) The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol 148:32–46. doi:10.1111/j.1365-2249.2007.03356.x
Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD (1997) Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 100:169–179. doi:10.1172/jci119509
Aggarwal BB (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3:745–756. doi:10.1038/nri1184
Anzai A, Anzai T, Nagai S, Maekawa Y, Naito K, Kaneko H, Sugano Y, Takahashi T, Abe H, Mochizuki S, Sano M, Yoshikawa T, Okada Y, Koyasu S, Ogawa S, Fukuda K (2012) Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation 125:1234–1245. doi:10.1161/circulationaha.111.052126
Avalos AM, Apablaza FA, Quiroz M, Toledo V, Pena JP, Michea L, Irarrazabal CE, Carrion FA, Figueroa FE (2012) IL-17A levels increase in the infarcted region of the left ventricle in a rat model of myocardial infarction. Biol Res 45:193–200. doi:10.1590/S0716-97602012000200012
Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA (2007) Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol 293:H1883–H1891. doi:10.1152/ajpheart.00514.2007
Bowers SL, Borg TK, Baudino TA (2010) The dynamics of fibroblast-myocyte-capillary interactions in the heart. Ann NY Acad Sci 1188:143–152. doi:10.1111/j.1749-6632.2009.05094.x
Brower GL, Janicki JS (2005) Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J Card Fail 11:548–556. doi:10.1016/j.cardfail.2005.05.005
Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M (2001) Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol 22:199–204. doi:10.1016/S1471-4906(01)01863-4
Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG (2008) Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol 173:57–67. doi:10.2353/ajpath.2008.070974
Cai ZP, Parajuli N, Zheng X, Becker L (2012) Remote ischemic preconditioning confers late protection against myocardial ischemia–reperfusion injury in mice by upregulating interleukin-10. Basic Res Cardiol 107:277. doi:10.1007/s00395-012-0277-1
Chandrasekar B, Mitchell DH, Colston JT, Freeman GL (1999) Regulation of CCAAT/enhancer binding protein, interleukin-6, interleukin-6 receptor, and gp130 expression during myocardial ischemia/reperfusion. Circulation 99:427–433. doi:10.1161/01.CIR.99.3.427
Colombo F, Gosselin H, El-Helou V, Calderone A (2003) Beta-adrenergic receptor-mediated DNA synthesis in neonatal rat cardiac fibroblasts proceeds via a phosphatidylinositol 3-kinase dependent pathway refractory to the antiproliferative action of cyclic AMP. J Cell Physiol 195:322–330. doi:10.1002/jcp.10251
Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, Barnes JL, Chandrasekar B (2007) IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol 293:H3356–H3365. doi:10.1152/ajpheart.00928.2007
D’Souza SP, Yellon DM, Martin C, Schulz R, Heusch G, Onody A, Ferdinandy P, Baxter GF (2003) B-type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel opening. Am J Physiol Heart Circ Physiol 284:H1592–H1600. doi:10.1152/ajpheart.00902.2002
Dörge H, Schulz R, Belosjorow S, Post H, van de Sand A, Konietzka I, Frede S, Hartung T, Vinten-Johansen J, Youker KA, Entman ML, Erbel R, Heusch G (2002) Coronary microembolization: the role of TNF-α in contractile dysfunction. J Mol Cell Cardiol 34:51–62. doi:10.1006/jmcc.2001.1489
Dean RG, Balding LC, Candido R, Burns WC, Cao Z, Twigg SM, Burrell LM (2005) Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem 53:1245–1256. doi:10.1369/jhc.4A6560.2005
Del Re DP, Matsuda T, Zhai P, Gao S, Clark GJ, Van Der Weyden L, Sadoshima J (2010) Proapoptotic Rassf1A/Mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice. J Clin Invest 120:3555–3567. doi:10.1172/JCI43569
Deten A, Holzl A, Leicht M, Barth W, Zimmer HG (2001) Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J Mol Cell Cardiol 33:1191–1207. doi:10.1006/jmcc.2001.1383
Deten A, Volz HC, Briest W, Zimmer HG (2002) Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc Res 55:329–340. doi:10.1016/S0008-6363(02)00413-3
Dhingra S, Sharma AK, Arora RC, Slezak J, Singal PK (2009) IL-10 attenuates TNF-alpha-induced NF kappaB pathway activation and cardiomyocyte apoptosis. Cardiovasc Res 82:59–66. doi:10.1093/cvr/cvp040
Divakaran V, Adrogue J, Ishiyama M, Entman ML, Haudek S, Sivasubramanian N, Mann DL (2009) Adaptive and maladaptive effects of SMAD3 signaling in the adult heart after hemodynamic pressure overloading. Circ Heart Fail 2:633–642. doi:10.1161/CIRCHEARTFAILURE.108.823070
Erlich JH, Boyle EM, Labriola J, Kovacich JC, Santucci RA, Fearns C, Morgan EN, Yun W, Luther T, Kojikawa O, Martin TR, Pohlman TH, Verrier ED, Mackman N (2000) Inhibition of the tissue factor-thrombin pathway limits infarct size after myocardial ischemia–reperfusion injury by reducing inflammation. Am J Pathol 157:1849–1862. doi:10.1016/S0002-9440(10)64824-9
Feng W, Li W, Liu W, Wang F, Li Y, Yan W (2009) IL-17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol-induced heart failure. Exp Mol Pathol 87:212–218. doi:10.1016/j.yexmp.2009.06.001
Feng Y, Hans C, McIlwain E, Varner KJ, Lazartigues E (2012) Angiotensin-converting enzyme 2 over-expression in the central nervous system reduces angiotensin-II-mediated cardiac hypertrophy. PLoS One 7:e48910. doi:10.1371/journal.pone.0048910
Fischer P, Hilfiker-Kleiner D (2007) Survival pathways in hypertrophy and heart failure: the gp130–STAT axis. Basic Res Cardiol 102:393–411. doi:10.1007/s00395-007-0674-z
Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML (2000) IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol 165:2798–2808
Fredj S, Bescond J, Louault C, Delwail A, Lecron JC, Potreau D (2005) Role of interleukin-6 in cardiomyocyte/cardiac fibroblast interactions during myocyte hypertrophy and fibroblast proliferation. J Cell Physiol 204:428–436. doi:10.1002/jcp.20307
Fredj S, Bescond J, Louault C, Potreau D (2005) Interactions between cardiac cells enhance cardiomyocyte hypertrophy and increase fibroblast proliferation. J Cell Physiol 202:891–899. doi:10.1002/Jcp.20197
Friedrichs GS, Swillo RE, Jow B, Bridal T, Numann R, Warner LM, Killar LM, Sidek K (2002) Sphingosine modulates myocyte electrophysiology, induces negative inotropy, and decreases survival after myocardial ischemia. J Cardiovasc Pharmacol 39:18–28. doi:10.1097/00005344-200201000-00003
Glenn DJ, Rahmutula D, Nishimoto M, Liang F, Gardner DG (2009) Atrial natriuretic peptide suppresses endothelin gene expression and proliferation in cardiac fibroblasts through a GATA4-dependent mechanism. Cardiovasc Res 84:209–217. doi:10.1093/cvr/cvp208
Golias C, Tsoutsi E, Matziridis A, Makridis P, Batistatou A, Charalabopoulos K (2007) Review. Leukocyte and endothelial cell adhesion molecules in inflammation focusing on inflammatory heart disease. In Vivo 21:757–769
Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS (1998) Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res 40:352–363. doi:10.1161/01.CIR.101.20.2338
Gruhle S, Sauter M, Szalay G, Ettischer N, Kandolf R, Klingel K (2012) The prostacyclin agonist iloprost aggravates fibrosis and enhances viral replication in enteroviral myocarditis by modulation of ERK signaling and increase of iNOS expression. Basic Res Cardiol 107:287. doi:10.1007/s00395-012-0287-z
Gulick T, Chung MK, Pieper SJ, Lange LG, Schreiner GF (1989) Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci USA 86:6753–6757. doi:10.1073/pnas.86.17.6753
Hao J, Wang B, Jones SC, Jassal DS, Dixon IMC (2000) Interaction between angiotensin II and Smad proteins in fibroblasts in failing heart and in vitro. Am J Physiol Heart Circ Physiol 279:H3020–H3030
Heine HL, Leong HS, Rossi FM, McManus BM, Podor TJ (2005) Strategies of conditional gene expression in myocardium: an overview. Methods Mol Med 112:109–154. doi:10.1007/978-1-59259-879-3_8
Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H (2007) A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128:589–600. doi:10.1016/j.cell.2006.12.036
Hu Y, Zhang H, Lu Y, Bai H, Xu Y, Zhu X, Zhou R, Ben J, Xu Y, Chen Q (2011) Class A scavenger receptor attenuates myocardial infarction-induced cardiomyocyte necrosis through suppressing M1 macrophage subset polarization. Basic Res Cardiol 106:1311–1328. doi:10.1007/s00395-011-0204-x
Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D (2009) Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell 16:233–244. doi:10.1016/j.devcel.2008.12.007
Jacobs M, Staufenberger S, Gergs U, Meuter K, Brandstatter K, Hafner M, Ertl G, Schorb W (1999) Tumor necrosis factor-alpha at acute myocardial infarction in rats and effects on cardiac fibroblasts. J Mol Cell Cardiol 31:1949–1959. doi:10.1006/jmcc.1999.1007
Jaffre F, Bonnin P, Callebert J, Debbabi H, Setola V, Doly S, Monassier L, Mettauer B, Blaxall BC, Launay JM, Maroteaux L (2009) Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy. Circ Res 104:113–123. doi:10.1161/CIRCRESAHA.108.180976
Joseph NM, Mosher JT, Buchstaller J, Snider P, McKeever PE, Lim M, Conway SJ, Parada LF, Zhu Y, Morrison SJ (2008) The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells. Cancer Cell 13:129–140. doi:10.1016/j.ccr.2008.01.003
Kakkar R, Lee RT (2010) Intramyocardial fibroblast myocyte communication. Circ Res 106:47–57. doi:10.1161/circresaha.109.207456
Kaneko K, Kanda T, Yokoyama T, Nakazato Y, Iwasaki T, Kobayashi I, Nagai R (1997) Expression of interleukin-6 in the ventricles and coronary arteries of patients with myocardial infarction. Res Commun Mol Pathol Pharmacol 97:3–12
Kao YH, Chen YC, Cheng CC, Lee TI, Chen YJ, Chen SA (2010) Tumor necrosis factor-alpha decreases sarcoplasmic reticulum Ca2+-ATPase expressions via the promoter methylation in cardiomyocytes. Crit Care Med 38:217–222. doi:10.1097/CCM.0b013e3181b4a854
Kapadia SR, Oral H, Lee J, Nakano M, Taffet GE, Mann DL (1997) Hemodynamic regulation of tumor necrosis factor-alpha gene and protein expression in adult feline myocardium. Circ Res 81:187–195. doi:10.1161/01.RES.81.2.187
Kapoun AM, Liang F, O’Young G, Damm DL, Quon D, White RT, Munson K, Lam A, Schreiner GF, Protter AA (2004) B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res 94:453–461. doi:10.1161/01.RES.0000117070.86556.9F
Kaur K, Sharma AK, Singal PK (2006) Significance of changes in TNF-alpha and IL-10 levels in the progression of heart failure subsequent to myocardial infarction. Am J Physiol Heart Circ Physiol 291:H106–H113. doi:10.1152/ajpheart.01327.2005
Kishimoto I, Rossi K, Garbers DL (2001) A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc Natl Acad Sci USA 98:2703–2706. doi:10.1073/pnas.051625598
Kleinbongard P, Heusch G, Schulz R (2010) TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther 127:295–314. doi:10.1016/j.pharmthera.2010.05.002
Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA (2011) Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest 121:2301–2312. doi:10.1172/JCI44824
Krown KA, Yasui K, Brooker MJ, Dubin AE, Nguyen C, Harris GL, McDonough PM, Glembotski CC, Palade PT, Sabbadini RA (1995) TNF alpha receptor expression in rat cardiac myocytes: TNF alpha inhibition of L-type Ca2+ current and Ca2+ transients. FEBS Lett 376:24–30. doi:10.1016/0014-5793(95)01238-5
Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, Glembotski CC, Quintana PJ, Sabbadini RA (1996) Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest 98:2854–2865. doi:10.1172/JCI119114
Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T (2004) Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension 43:739–745. doi:10.1161/01.HYP.0000118584.33350.7d
Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Dechend R, Luft FC, Muller DN (2009) Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 119:2904–2912. doi:10.1161/CIRCULATIONAHA.108.832782
LaFramboise WA, Scalise D, Stoodley P, Graner SR, Guthrie RD, Magovern JA, Becich MJ (2007) Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am J Physiol Cell Physiol 292:C1799–C1808. doi:10.1152/ajpcell.00166.2006
Levine B, Kalman J, Mayer L, Fillit HM, Packer M (1990) Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323:236–241. doi:10.1056/NEJM199007263230405
Liao CH, Akazawa H, Tamagawa M, Ito K, Yasuda N, Kudo Y, Yamamoto R, Ozasa Y, Fujimoto M, Wang P, Nakauchi H, Nakaya H, Komuro I (2010) Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest 120:242–253. doi:10.1172/JCI39942
Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, Saffitz J, Chien K, Xiao RP, Kass DA, Wang Y (2001) The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci USA 98:12283–12288. doi:10.1073/pnas.211086598
Liao YH, Xia N, Zhou SF, Tang TT, Yan XX, Lv BJ, Nie SF, Wang J, Iwakura Y, Xiao H, Yuan J, Jevallee H, Wei F, Shi GP, Cheng X (2012) Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol 59:420–429. doi:10.1016/j.jacc.2011.10.863
Long CS, Hartogensis WE, Simpson PC (1993) Beta-adrenergic stimulation of cardiac non-myocytes augments the growth-promoting activity of non-myocyte conditioned medium. J Mol Cell Cardiol 25:915–925. doi:10.1006/jmcc.1993.1104
Mann DL (2002) Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 91:988–998. doi:10.1161/01.RES.0000043825.01705.1B
Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T (2004) Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 109:1594–1602. doi:10.1161/01.CIR.0000124490.27666.B2
Marra F, Aleffi S, Galastri S, Provenzano A (2009) Mononuclear cells in liver fibrosis. Semin Immunopathol 31:345–358. doi:10.1007/s00281-009-0169-0
Matsui Y, Sadoshima J (2004) Rapid upregulation of CTGF in cardiac myocytes by hypertrophic stimuli: implication for cardiac fibrosis and hypertrophy. J Mol Cell Cardiol 37:477–481. doi:10.1016/j.yjmcc.2004.05.012
McMullen JR (2008) Role of insulin-like growth factor 1 and phosphoinositide 3-kinase in a setting of heart disease. Clin Exp Pharmacol Physiol 35:349–354. doi:10.1111/j.1440-1681.2007.04873.x
McTiernan CF, Lemster BH, Frye C, Brooks S, Combes A, Feldman AM (1997) Interleukin-1 beta inhibits phospholamban gene expression in cultured cardiomyocytes. Circ Res 81:493–503. doi:10.1161/01.RES.81.4.493
Miller C, Cai Y, Oikawa M, Thomas T, Dostmann W, Zaccolo M, Fujiwara K, Yan C (2011) Cyclic nucleotide phosphodiesterase 1A: a key regulator of cardiac fibroblast activation and extracellular matrix remodeling in the heart. Basic Res Cardiol 106:1023–1039. doi:10.1007/s00395-011-0228-2
Mitchell MD, Laird RE, Brown RD, Long CS (2007) IL-1beta stimulates rat cardiac fibroblast migration via MAP kinase pathways. Am J Physiol Heart Circ Physiol 292:H1139–H1147. doi:10.1152/ajpheart.00881.2005
Murray DR, Prabhu SD, Chandrasekar B (2000) Chronic beta-adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation 101:2338–2341. doi:10.1161/01.CIR.101.20.2338
Novoyatleva T, Schymura Y, Janssen W, Strobl F, Swiercz JM, Patra C, Posern G, Wietelmann A, Zheng TS, Schermuly RT, Engel FB (2013) Deletion of Fn14 receptor protects from right heart fibrosis and dysfunction. Basic Res Cardiol 108:325. doi:10.1007/s00395-012-0325-x
Parajuli N, Yuan Y, Zheng X, Bedja D, Cai Z (2012) Phosphatase PTEN is critically involved in post-myocardial infarction remodeling through the Akt/interleukin-10 signaling pathway. Basic Res Cardiol 107:248. doi:10.1007/s00395-012-0248-6
Pawlinski R, Tencati M, Hampton CR, Shishido T, Bullard TA, Casey LM, Andrade-Gordon P, Kotzsch M, Spring D, Luther T, Abe J, Pohlman TH, Verrier ED, Blaxall BC, Mackman N (2007) Protease-activated receptor-1 contributes to cardiac remodeling and hypertrophy. Circulation 116:2298–2306. doi:10.1161/CIRCULATIONAHA.107.692764
Pedrotty DM, Klinger RY, Kirkton RD, Bursac N (2009) Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovasc Res 83:688–697. doi:10.1093/Cvr/Cvp164
Peng H, Yang X-P, Carretero OA, Nakagawa P, D’Ambrosio M, Leung P, Xu J, Peterson EL, González GE, Harding P, Rhaleb N-E (2011) Angiotensin II-induced dilated cardiomyopathy in Balb/c but not C57BL/6J mice. Exp Physiol 96:756–764. doi:10.1113/expphysiol.2011.057612
Podewski EK, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, Drexler H (2003) Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation 107:798–802. doi:10.1161/01.CIR.0000057545.82749.FF
Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X (2007) The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 8:247–256. doi:10.1038/ni1439
Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD (2000) Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 102:3060–3067. doi:10.1161/01.CIR.102.25.3060
Sano M, Fukuda K, Kodama H, Pan J, Saito M, Matsuzaki J, Takahashi T, Makino S, Kato T, Ogawa S (2000) Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem 275:29717–29723. doi:10.1074/jbc.M003128200
Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I (2007) p53-Induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446:444–448. doi:10.1038/nature05602
Schultz Jel J, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T (2002) TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest 109:787–796. doi:10.1172/JCI14190
Schulz R, Panas DL, Catena R, Moncada S, Olley PM, Lopaschuk GD (1995) The role of nitric oxide in cardiac depression induced by interleukin-1 beta and tumour necrosis factor-alpha. Br J Pharmacol 114:27–34. doi:10.1111/j.1476-5381.1995.tb14901.x
Shindo T, Manabe I, Fukushima Y, Tobe K, Aizawa K, Miyamoto S, Kawai-Kowase K, Moriyama N, Imai Y, Kawakami H, Nishimatsu H, Ishikawa T, Suzuki T, Morita H, Maemura K, Sata M, Hirata Y, Komukai M, Kagechika H, Kadowaki T, Kurabayashi M, Nagai R (2002) Kruppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat Med 8:856–863. doi:10.1038/nm738
Siwik DA, Chang DL, Colucci WS (2000) Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res 86:1259–1265. doi:10.1161/01.RES.86.12.1259
Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, Heusch G (2007) Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res 100:140–146. doi:10.1161/01.RES.0000255031.15793.86
Sobirin MA, Kinugawa S, Takahashi M, Fukushima A, Homma T, Ono T, Hirabayashi K, Suga T, Azalia P, Takada S, Taniguchi M, Nakayama T, Ishimori N, Iwabuchi K, Tsutsui H (2012) Activation of natural killer T cells ameliorates postinfarct cardiac remodeling and failure in mice. Circ Res 111:1037–1047. doi:10.1161/CIRCRESAHA.112.270132
Sperr WR, Bankl HC, Mundigler G, Klappacher G, Grossschmidt K, Agis H, Simon P, Laufer P, Imhof M, Radaszkiewicz T, Glogar D, Lechner K, Valent P (1994) The human cardiac mast cell: localization, isolation, phenotype, and functional characterization. Blood 84:3876–3884
Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, Liu PP (2004) Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation 110:3221–3228. doi:10.1161/01.CIR.0000147233.10318.23
Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo J-L, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325:612–616. doi:10.1126/science.1175202
Swirski FK, Nahrendorf M (2013) Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339:161–166. doi:10.1126/science.1230719
Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, Conway SJ, Nagai R (2010) Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest 120:254–265. doi:10.1172/Jci40295
Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, Yan XX, Nie SF, Liu J, Zhou SF, Li JJ, Yao R, Liao MY, Tu X, Liao YH, Cheng X (2012) Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol 107:232. doi:10.1007/s00395-011-0232-6
Thielmann M, Dörge H, Martin C, Belosjorow S, Schwanke U, van de Sand A, Konietzka I, Büchert A, Krüger A, Schulz R, Heusch G (2002) Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-α, and sphingosine. Circ Res 90:807–813. doi:10.1161/01.res.0000014451.75415.36
Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL (1996) Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 27:1201–1206. doi:10.1016/0735-1097(95)00589-7
Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL (1996) Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation 93:704–711. doi:10.1161/01.CIR.93.4.704
Tsutsumi Y, Matsubara H, Ohkubo N, Mori Y, Nozawa Y, Murasawa S, Kijima K, Maruyama K, Masaki H, Moriguchi Y, Shibasaki Y, Kamihata H, Inada M, Iwasaka T (1998) Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ Res 83:1035–1046. doi:10.1161/01.RES.83.10.1035
Turner NA, Porter KE, Smith WH, White HL, Ball SG, Balmforth AJ (2003) Chronic beta2-adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovasc Res 57:784–792. doi:10.1016/S0008-6363(02)00729-0
Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schutz G, Lumeng CN, Mortensen RM (2010) Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest 120:3350–3364. doi:10.1172/JCI41080
van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ (2007) Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 170:818–829. doi:10.2353/ajpath.2007.060547
Venkatachalam K, Mummidi S, Cortez DM, Prabhu SD, Valente AJ, Chandrasekar B (2008) Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. Am J Physiol Heart Circ Physiol 294:H2078–H2087. doi:10.1152/ajpheart.01363.2007
Volz HC, Laohachewin D, Seidel C, Lasitschka F, Keilbach K, Wienbrandt AR, Andrassy J, Bierhaus A, Kaya Z, Katus HA, Andrassy M (2012) S100A8/A9 aggravates post-ischemic heart failure through activation of RAGE-dependent NF-kappaB signaling. Basic Res Cardiol 107:250. doi:10.1007/s00395-012-0250-z
Wang J, Xu N, Feng X, Hou N, Zhang J, Cheng X, Chen Y, Zhang Y, Yang X (2005) Targeted disruption of Smad4 in cardiomyocytes results in cardiac hypertrophy and heart failure. Circ Res 97:821–828. doi:10.1161/01.RES.0000185833.42544.06
Wei L (2011) Immunological aspect of cardiac remodeling: T lymphocyte subsets in inflammation-mediated cardiac fibrosis. Exp Mol Pathol 90:74–78. doi:10.1016/j.yexmp.2010.10.004
Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschope C (2011) Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 4:44–52. doi:10.1161/CIRCHEARTFAILURE.109.931451
Yamaoka M, Yamaguchi S, Okuyama M, Tomoike H (1999) Anti-inflammatory cytokine profile in human heart failure: behavior of interleukin-10 in association with tumor necrosis factor-alpha. Jpn Circ J 63:951–956. doi:10.1253/jcj.63.951
Yamazaki T, Komuro I, Yazaki Y (1998) Signalling pathways for cardiac hypertrophy. Cell Signal 10:693–698. doi:10.1016/S0898-6568(98)00036-9
Yokoyama T, Nakano M, Bednarczyk JL, McIntyre BW, Entman M, Mann DL (1997) Tumor necrosis factor-alpha provokes a hypertrophic growth response in adult cardiac myocytes. Circulation 95:1247–1252. doi:10.1161/01.CIR.95.5.1247
Yoon PO, Lee MA, Cha H, Jeong MH, Kim J, Jang SP, Choi BY, Jeong D, Yang DK, Hajjar RJ, Park WJ (2010) The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J Mol Cell Cardiol 49:294–303. doi:10.1016/j.yjmcc.2010.04.010
Yu Q, Horak K, Larson DF (2006) Role of T lymphocytes in hypertension-induced cardiac extracellular matrix remodeling. Hypertension 48:98–104. doi:10.1161/01.HYP.0000227247.27111.b2
Zaglia T, Milan G, Franzoso M, Bertaggia E, Pianca N, Piasentini E, Voltarelli VA, Chiavegato D, Brum PC, Glass DJ, Schiaffino S, Sandri M, Mongillo M (2013) Cardiac sympathetic neurons provide trophic signal to the heart via beta2-adrenoceptor-dependent regulation of proteolysis. Cardiovasc Res 97:240–250. doi:10.1093/cvr/cvs320
Zamilpa R, Kanakia R, Cigarroa J 4th, Dai Q, Escobar GP, Martinez H, Jimenez F, Ahuja SS, Lindsey ML (2011) CC chemokine receptor 5 deletion impairs macrophage activation and induces adverse remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 300:H1418–H1426. doi:10.1152/ajpheart.01002.2010
Zandbergen HR, Sharma UC, Gupta S, Verjans JW, van den Borne S, Pokharel S, van Brakel T, Duijvestijn A, van Rooijen N, Maessen JG, Reutelingsperger C, Pinto YM, Narula J, Hofstra L (2009) Macrophage depletion in hypertensive rats accelerates development of cardiomyopathy. J Cardiovasc Pharmacol Ther 14:68–75. doi:10.1177/1074248408329860
Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13:952–961. doi:10.1038/nm1613
Zhang D, Gaussin V, Taffet GE, Belaguli NS, Yamada M, Schwartz RJ, Michael LH, Overbeek PA, Schneider MD (2000) TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med 6:556–563. doi:10.1038/75037
Zhong Z, Wen Z, Darnell JE Jr (1994) Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264:95–98. doi:10.1126/science.8140422
Zucker IH, Liu JL (2000) Angiotensin II–nitric oxide interactions in the control of sympathetic outflow in heart failure. Heart Fail Rev 5:27–43. doi:10.1023/A:1009894007055
Acknowledgments
This study was supported by the “Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program)” (to R.N.), Grants-in-Aid for Scientific Research (S) and (B), and Grants-in-Aid for Young Scientists (B) from JSPS (23390203, 22229006, 23790835) (to R.N., K.F.); a grant for Translational Systems Biology and Medicine Initiative (to R.N.) from JST.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is part of the Topical Collection Novel Perspectives on Heart Failure.
Rights and permissions
About this article
Cite this article
Fujiu, K., Nagai, R. Contributions of cardiomyocyte–cardiac fibroblast–immune cell interactions in heart failure development. Basic Res Cardiol 108, 357 (2013). https://doi.org/10.1007/s00395-013-0357-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-013-0357-x