Abstract
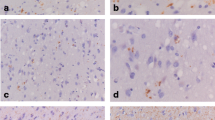
Alzheimer’s disease (AD) is, at the neuropathological level, characterized by the accumulation and aggregation of misfolded proteins. The presence of misfolded proteins in the endoplasmic reticulum (ER) triggers a cellular stress response called the unfolded protein response (UPR) that may protect the cell against the toxic buildup of misfolded proteins. In this study we investigated the activation of the UPR in AD. Protein levels of BiP/GRP78, a molecular chaperone which is up-regulated during the UPR, was found to be increased in AD temporal cortex and hippocampus as determined by Western blot analysis. At the immunohistochemical level intensified staining of BiP/GRP78 was observed in AD, which did not co-localize with AT8-positive neurofibrillary tangles. In addition, we performed immunohistochemistry for phosphorylated (activated) pancreatic ER kinase (p-PERK), an ER kinase which is activated during the UPR. p-PERK was observed in neurons in AD patients, but not in non-demented control cases and did not co-localize with AT8-positive tangles. Overall, these data show that the UPR is activated in AD, and the increased occurrence of BiP/GRP78 and p-PERK in cytologically normal-appearing neurons suggest a role for the UPR early in AD neurodegeneration. Although the initial participation of the UPR in AD pathogenesis might be neuroprotective, sustained activation of the UPR in AD might initiate or mediate neurodegeneration.




Similar content being viewed by others
References
Benn SC, Woolf CJ (2004) Adult neuron survival strategies—slamming on the brakes. Nat Rev Neurosci 5:686–700
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–8; discussion 278–284
Chang RC, Wong AK, Ng HK, Hugon J (2002) Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer’s disease. Neuroreport 13:2429–2432
Cook DG, Forman MS, Sung JC, Leight S, Kolson DL, Iwatsubo T, Lee VM, Doms RW (1997) Alzheimer’s A beta(1–42) is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nat Med 3:1021–1023
Forman MS, Lee VM, Trojanowski JQ (2003) ‘Unfolding’ pathways in neurodegenerative disease. Trends Neurosci 26:407–410
Hamos JE, Oblas B, Pulaski-Salo D, Welch WJ, Bole DG, Drachman DA (1991) Expression of heat shock proteins in Alzheimer’s disease. Neurology 41:345–350
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274
Hartmann T, Bieger SC, Bruhl B, Tienari PJ, Ida N, Allsop D, Roberts GW, Masters CL, Dotti CG, Unsicker K, Beyreuther K (1997) Distinct sites of intracellular production for Alzheimer’s disease A beta40/42 amyloid peptides. Nat Med 3:1016–1020
Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, Hitomi J, Morihara T, Yoneda T, Gomi F, Mori Y, Nakano Y, Takeda J, Tsuda T, Itoyama Y, Murayama O, Takashima A, St George-Hyslop P, Takeda M, Tohyama M (1999) Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat Cell Biol 1:479–485
Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J (1988) The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332:462–464
Onuki R, Bando Y, Suyama E, Katayama T, Kawasaki H, Baba T, Tohyama M, Taira K (2004) An RNA-dependent protein kinase is involved in tunicamycin-induced apoptosis and Alzheimer’s disease. EMBO J 23:959–968
Piccini A, Fassio A, Pasqualetto E, Vitali A, Borghi R, Palmieri D, Nacmias B, Sorbi S, Sitia R, Tabaton M (2004) Fibroblasts from FAD-linked presenilin 1 mutations display a normal unfolded protein response but overproduce Abeta42 in response to tunicamycin. Neurobiol Dis 15:380–386
Reisberg B, Ferris SH, Leon MJ de, Crook T (1982) The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 139:1136–1139
Rutkowski DT, Kaufman RJ (2004) A trip to the ER: coping with stress. Trends Cell Biol 14:20–28
Sato N, Urano F, Yoon Leem J, Kim SH, Li M, Donoviel D, Bernstein A, Lee AS, Ron D, Veselits ML, Sisodia SS, Thinakaran G (2000) Upregulation of BiP and CHOP by the unfolded-protein response is independent of presenilin expression. Nat Cell Biol 2:863–870
Scheper W, Hol E (2005) Protein quality control in Alzheimer’s disease: a fatal saviour. Curr Drug Targets CNS Neurol Disord 4:283–292
Suen KC, Lin KF, Elyaman W, So KF, Chang RC, Hugon J (2003) Reduction of calcium release from the endoplasmic reticulum could only provide partial neuroprotection against beta-amyloid peptide toxicity. J Neurochem 87:1413–1426
Suen KC, Yu MS, So KF, Chang RC, Hugon J (2003) Upstream signaling pathways leading to the activation of double-stranded RNA-dependent serine/threonine protein kinase in beta-amyloid peptide neurotoxicity. J Biol Chem 278:49819–49827
Taylor JP, Hardy J, Fischbeck KH (2002) Toxic proteins in neurodegenerative disease. Science 296:1991–1995
Yamaguchi H, Haga C, Hirai S, Nakazato Y, Kosaka K (1990) Distinctive, rapid, and easy labeling of diffuse plaques in the Alzheimer brains by a new methenamine silver stain. Acta Neuropathol 79:569–572
Yu Z, Luo H, Fu W, Mattson MP (1999) The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol 155:302–314
Acknowledgements
The authors thank the Netherlands Brain Bank for supplying the human brain tissue (coordinator Dr. R. Ravid), Dr. W. Kamphorst for the neuropathological diagnosis of control and AD tissue, and Marlies Jacobs for her technical assistance. This study was supported by the Internationale Stichting Alzheimer Onderzoek (ISAO grant 02501 to J.H., grant 02504 to W.S.). W.S. is a fellow of the Anton Meelmeijer Center for Translational Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoozemans, J.J.M., Veerhuis, R., Van Haastert, E.S. et al. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol 110, 165–172 (2005). https://doi.org/10.1007/s00401-005-1038-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-005-1038-0