Abstract
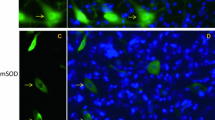
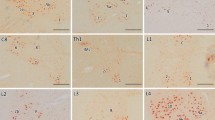
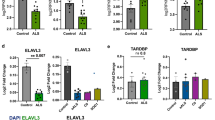
Recent investigations have indicated that the nucleocytoplasmic transport system is essential for maintaining cell viability and cellular functions and that its dysfunction could lead to certain disorders. To investigate the involvement of this system in the pathomechanisms of amyotrophic lateral sclerosis (ALS), we examined the immunohistochemical localization of proteins associated with nucleocytoplasmic transport in the lumbar spinal cord in a mutant SOD1 (G93A) transgenic mouse model of ALS. This model is widely used for ALS research, and the mutant mice are known to exhibit neuronal loss and Lewy body-like hyaline inclusions (LBHIs) in the anterior horns, similar to the pathology seen in familial ALS patients associated with an SOD1 mutation and in several other transgenic rodent models. Using antibodies against the importin beta family of proteins, the major carrier proteins of nucleocytoplasmic transport, and those against their adapter protein, importin alpha, we found that the immunoreactivities were decreased within the nuclei and increased within the cytoplasm of a subset of the surviving anterior horn cells of the transgenic mice. In addition, LBHIs were invariably reactive toward these antibodies. Furthermore, the immunoreactivities for histone H1 and beta-catenin, representative cargo proteins transported by importin beta-dependent and beta-independent nucleocytoplasmic transport pathways, respectively, showed distributions similar to those for importin beta family and importin alpha proteins. The altered distributions of these proteins were not associated with caspase-3 expression, suggesting that the findings are unlikely to be a manifestation of apoptotic processes. Chronological quantitative analysis of importin beta-immunostained sections from the transgenic mice revealed a statistically significant progressive decrease in the proportion of the anterior horn cells exhibiting a more intense reactivity for these proteins in the nucleus than in the cytoplasm. To the contrary, we found that the anterior horn cells with the immunoreactivity in their cytoplasm, being more pronounced than that in their nucleus, were significantly increased in number along with the disease progression. This is the first report investigating nucleocytoplasmic transport in the ALS model mouse, and our present results imply that its dysfunction could be involved in the pathomechanisms underlying ALS.


Similar content being viewed by others
References
Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW (1997) ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18:327–338
Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW (1998) Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 281:1851–1854
Cronshaw JM, Matunis MJ (2004) The nuclear pore complex: disease associations and functional correlations. Trends Endocrinol Metab 15:34–39
Dal Canto MC, Gurney ME (1994) Development of central nervous system pathology in a murine transgenic model of human amyotrophic lateral sclerosis. Am J Pathol 145:1271–1279
Dal Canto MC, Gurney ME (1995) Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS). Brain Res 676:25–40
Faleiro L, Lazebnik Y (2000) Caspases disrupt the nuclear-cytoplasmic barrier. J Cell Biol 151:951–959
Feng H, Zhong W, Punkosdy G, Gu S, Zhou L, Seabolt EK, Kipreos ET (1999) CUL-2 is required for the G1-to-S-phase transition and mitotic chromosome condensation in Caenorhabditis elegans. Nat Cell Biol 1:486–492
Fried H, Kutay U (2003) Nucleocytoplasmic transport: taking an inventory. Cell Mol Life Sci 60:1659–1688
Ghanevati M, Miller CA (2005) Phospho-β-catenin accumulation in Alzheimer’s disease and in aggresomes attributable to proteasome dysfunction. J Mol Neurosci 25:79–94
Görlich D, Kutay U (1999) Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol 15:607–660
Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M (2003) Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40:1095–1104
Hirano A, Kurland LT, Sayre GP (1967) Familial amyotrophic lateral sclerosis. A subgroup characterized by posterior and spinocerebellar tract involvement and hyaline inclusions in the anterior horn cells. Arch Neurol 16:232–243
Hu JH, Chernoff K, Pelech S, Krieger C (2003) Protein kinase and protein phosphatase expression in the central nervous system of G93A mSOD over-expressing mice. J Neurochem 85:422–431
Jäkel S, Albig W, Kutay U, Bischoff FR, Schwamborn K, Doenecke D, Görlich D (1999) The importin β/importin 7 heterodimer is a functional nuclear import receptor for histone H1. EMBO J 18:2411–2423
Kabashi E, Agar JN, Taylor DM, Minotti S, Durham HD (2004) Focal dysfunction of the proteasome: a pathogenic factor in a mouse model of amyotrophic lateral sclerosis. J Neurochem 89:1325–1335
Köhler M, Speck C, Christiansen M, Bischoff FR, Prehn S, Haller H, Görlich D, Hartmann E (1999) Evidence for distinct substrate specificities of importin α family members in nuclear protein import. Mol Cell Biol 19:7782–7791
Koike M, Kose S, Furuta M, Taniguchi N, Yokoya F, Yoneda Y, Imamoto N (2004) beta-Catenin shows an overlapping sequence requirement but distinct molecular interactions for its bidirectional passage through nuclear pores. J Biol Chem 279: 34038–34047
Kuersten S, Ohno M, Mattaj IW (2001) Nucleocytoplasmic transport: Ran, beta and beyond. Trends Cell Biol 11:497–503
Loveland KL, Hogarth C, Szczepny A, Prabhu SM, Jans DA (2006) Expression of nuclear transport importins beta 1 and beta 3 is regulated during rodent spermatogenesis. Biol Reprod 74:67–74
Lucas JJ, Hernández F, Gómez-Ramos P, Morán MA, Hen R, Avila J (2001) Decreased nuclear β-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3β conditional transgenic mice. EMBO J 20:27–39
Moroianu J, Hijikata M, Blobel G, Radu A (1995) Mammalian karyopherin α1β and α2β heterodimers: α1 or α2 subunit binds nuclear localization signal and β subunit interacts with peptide repeat-containing nucleoporins. Proc Natl Acad Sci USA 92:6532–6536
Mosammaparast N, Pemberton LF (2004) Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol 14:547–556
Ribbeck K, Lipowsky G, Kent HM, Stewart M, Görlich D (1998) NTF2 mediates nuclear import of Ran. EMBO J 17:6587–6598
Rollenhagen C, Muhlhausser P, Kutay U, Panté N (2003) Importin β-depending nuclear import pathways: role of the adapter proteins in the docking and releasing steps. Mol Biol Cell 14:2104–2115
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung WY, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown Jr RH (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62
Rout MP, Aitchison JD (2001) The nuclear pore complex as a transport machine. J Biol Chem 276:16593–16596
Salman H, Abu-Arish A, Oliel S, Loyter A, Klafter J, Granek R, Elbaum M (2005) Nuclear localization signal peptides induce molecular delivery along microtubules. Biophys J 89:2134–2145
Sheffield LG, Miskiewicz HB, Tannenbaum LB, Mirra SS (2006) Nuclear pore complex proteins in Alzheimer disease. J Neuropathol Exp Neurol 65:45–54
Suntharalingam M, Wente SR (2003) Peering through the pore: nuclear pore complex structure, assembly, and function. Dev Cell 4:775–789
Um JW, Min DS, Rhim H, Kim J, Paik SR, Chung KC (2006) Parkin ubiquitinates and promotes the degradation of RanBP2. J Biol Chem 281:3595–3603
Watanabe M, Dykes-Hoberg M, Culotta VC, Price DL, Wong PC, Rothstein JD (2001) Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues. Neurobiol Dis 8:933–941
Wate R, Ito H, Zhang JH, Ohnishi S, Nakano S, Kusaka H (2005) Expression of an endoplasmic reticulum-resident chaperone, glucose-regulated stress protein 78, in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Acta Neuropathol 110:557–562
Wengenack TM, Holasek SS, Montano CM, Gregor D, Curran GL, Poduslo JF (2004) Activation of programmed cell death markers in ventral horn motor neurons during early presymptomatic stages of amyotrophic lateral sclerosis in a transgenic mouse model. Brain Res 1027:73–86
Acknowledgments
We express our sincere appreciation to Professor Asao Hirano (Division of Neuropathology, Department of Pathology, Montefiore Medical Center) for helpful comments, and to Miss Tomoko Takemi for her technical assistance. This work was supported in part by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (No. 15590917) and by a grant from Kansai Medical University (Research Grant B, 2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Ito, H., Wate, R. et al. Altered distributions of nucleocytoplasmic transport-related proteins in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Acta Neuropathol 112, 673–680 (2006). https://doi.org/10.1007/s00401-006-0130-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0130-4