Abstract
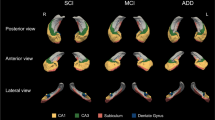
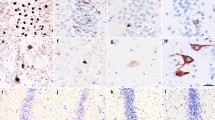
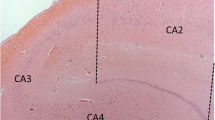
The hippocampal involvement in amyotrophic lateral sclerosis (ALS) patients has been known for more than a decade, however, its relationship to clinical manifestations including memory deficits and topographical differentiation from Alzheimer disease (AD) remain unclear. In order to clarify the anatomopathological features in the hippocampus and their relevance to disease-specific memory deficits in ALS patients, topography and cytopathology of the hippocampal lesions along the perforant pathway were quantitatively and semiquantitatively surveyed in 14 ALS patients with extramotor involvement. These pathological findings were compared with clinical characteristics assessed from their clinical records. Cytoplasmic inclusions initially appear in the granular cells of the dentate gyrus (DG) and superficial small neurons of the transentorhinal cortex (TEC) with mild subicular degeneration (stage I: inclusion stage). Subsequent gliosis and neuronal loss of the TEC, concomitant with presynaptic degeneration of the outer molecular layer of the DG, suggests an extension of the degeneration through the perforant pathway (stage II: early perforant stage). In a more advanced stage, the presynaptic degeneration is more evident with moderate to severe neuronal loss in the TEC (stage III: advanced perforant stage). This advanced stage was associated with episodic memory deficits mimicking AD in some ALS patients. This ALS pathology initiated by cytoplasmic inclusions and neuronal loss in layer II–III of the TEC is different from neurofibrillary tangles of AD, dominant in layer II–III of the entorhinal cortex. Because this involvement of the TEC-molecular DG projection and subiculum is specific to ALS, it will provide a basis for clinical characterization of memory deficits of ALS, which could be distinct from those of AD.





Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer disease
- ALS:
-
Amyotrophic lateral sclerosis
- ALSD:
-
Amyotrophic lateral sclerosis with dementia
- CA:
-
Cornu ammonis
- DG:
-
Dentate gyrus
- EC:
-
Entorhinal cortex
- FTD/MND:
-
Frontotemporal dementia with motor neuron disease
- NFTs:
-
Neurofibrillary tangles
- TEC:
-
Transentorhinal cortex
- UTCIs:
-
Ubiquitin/TDP-43-positive cytoplasmic inclusions
References
Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW (2007) Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol (Berl) 113:245–252
Arai T, Hasegawa M, Akiyama H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Braak H (1980) Architechtonics of human telencephalic cortex. Springer, Berlin
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 82:239–259
Braak H, Braak E (1992) The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neurosci Res 15:6–31
Cairns NJ, Neumann M, Bigio EH et al (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240
Damasio AR (1984) The anatomic basis of memory disorders. Semin Neurol 4:226–228
Davies P (1992) Alz 50 as a reagent to assess animal models of Alzheimer’s disease. Neurobiol Aging 13:613–614
Dickson DW, Ruan D, Crystal H et al (1991) Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer’s disease: light and electron microscopic immunocytochemistry of CA2-3 neurites specific to DLBD. Neurology 41:1402–1409
Duyckaerts C, Colle MA, Seilhean D, Hauw JJ (1998) Laminar spongiosis of the dentate gyrus: a sign of disconnection, present in cases of severe Alzheimer’s disease. Acta Neuropathol (Berl) 95:413–420
Duyckaerts C, Potier MC, Delatour B (2008) Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol (Berl) 115:5–38
Garey (1999) Common features in cortical architectonics. Brodman’s ‘Localization in the cerebral cortex’. Imperial College Press, London, pp 107–174
Graham A, Davies R, Xuereb J et al (2005) Pathologically proven frontotemporal dementia presenting with severe amnesia. Brain 128:597–605
Hjorth-Simonsen A, Jeune B (1972) Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J Comp Neurol 144:215–232
Hodges JR, Davies RR, Xuereb JH et al (2004) Clinicopathological correlates in frontotemporal dementia. Ann Neurol 56:399–406
Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR (1986) Perforant pathway changes and the memory impairment of Alzheimer’s disease. Ann Neurol 20:472–481
Insausti R, Amaral DG (2004) Hippocampal formation. In: Paxinos G, Mai J (eds) The human nervous system. Academic Press, New York, pp 871–914
Ishizuka N, Weber J, Amaral DG (1990) Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol 295:580–623
Jackson M, Lennox G, Lowe J (1996) Motor neurone disease-inclusion dementia. Neurodegeneration 5:339–350
Josephs KA, Lin WL, Ahmed Z, Stroh DA, Graff-Radford NR, Dickson DW (2008) Frontotemporal lobar degeneration with ubiquitin-positive, but TDP-43-negative inclusions. Acta Neuropathol 116:159–167
Lakerveld J, Kotchoubey B, Kubler A (2008) Cognitive function in patients with late stage amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 79:25–29
Lippa CF (2004) Synaptophysin immunoreactivity in Pick’s disease: comparison with Alzheimer’s disease and dementia with Lewy bodies. Am J Alzheimers Dis Other Demen 19:341–344
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944
Mizutani T, Kasahara M (1995) Degeneration of the intrahippocampal routes of the perforant and alvear pathways in senile dementia of Alzheimer type. Neurosci Lett 184:141–144
Nakano I, Iwatsubo T, Hashizume Y, Mizutani T, Mannen T (1992) Amyotrophic lateral sclerosis with dementia lesions in the apical cortex and some deeper structures of the temporal lobes. Neuropathology 12:69–77
Neary D, Snowden JS, Gustafson L et al (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Neary D, Snowden JS, Mann DM, Northen B, Goulding PJ, Macdermott N (1990) Frontal lobe dementia and motor neuron disease. J Neurol Neurosurg Psychiatry 53:23–32
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Nishihira Y, Tan CF, Onodera O et al (2008) Sporadic amyotrophic lateral sclerosis: two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathol 116:169–182
Okamoto K, Hirai S, Yamazaki T, Sun XY, Nakazato Y (1991) New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett 129:233–236
Oyler GA, Higgins GA, Hart RA et al (1989) The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol 109:3039–3052
Squire LR, Zola-Morgan S (1983) The neurology of memory: the case for correspondance between the findings for human and nonhuman primate. Academic Press, New York
Takeda T, Uchihara T, Mochizuki Y, Mizutani T, Iwata M (2007) Memory deficits in amyotrophic lateral sclerosis patients with dementia and degeneration of the perforant pathway. A clinicopathological study. J Neurol Sci 260:225–230
Tolnay M, Schwietert M, Monsch AU, Staehelin HB, Langui D, Probst A (1997) Argyrophilic grain disease: distribution of grains in patients with and without dementia. Acta Neuropathol 94:353–358
Uchihara T, Nakamura A, Nakayama H et al (2003) Triple immunofluorolabeling with two rabbit polyclonal antibodies and a mouse monoclonal antibody allowing three-dimensional analysis of cotton wool plaques in Alzheimer disease. J Histochem Cytochem 51:1201–1206
Uchihara T, Tsuchiya K (2008) Neuropathology of Pick body disease. In: Duyckaerts C, Litvan I (eds) Handbook of clinical neurology. Elsevier, Amsterdam, pp 395–410
Van Hoesen GW, Pandya DN (1975) Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Res 95:39–59
West MJ, Slomianka L (1998) Total number of neurons in the layers of the human entorhinal cortex. Hippocampus 8:69–82
Wightman G, Anderson VE, Martin J et al (1992) Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett 139:269–274
Witter MP, Amaral DG (1991) Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J Comp Neurol 307:437–459
Acknowledgments
We thank Dr. Norio Ishizuka, Department of Brain Structure, Tokyo Metropolitan Institute for Neuroscience, Dr. Yoko Mochizuki, Department of Pathology, Tokyo Metropolitan Neurological Hospital, Dr. Naoto Uyama, Department of Neurology, Tokyo Metropolitan Neurological Hospital and Dr. Masako Yamazaki, Department of Neurology, Tokyo Women’s Medical University for their assistance and advice for clinicopathological analyses. We are also grateful to Mr. Yoshitomo Umitsu and Ms. Ayako Nakamura for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeda, T., Uchihara, T., Arai, N. et al. Progression of hippocampal degeneration in amyotrophic lateral sclerosis with or without memory impairment: distinction from Alzheimer disease. Acta Neuropathol 117, 35–44 (2009). https://doi.org/10.1007/s00401-008-0447-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0447-2