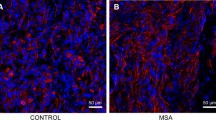
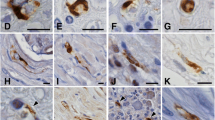
Abstract
Involvement of the peripheral nervous system (PNS) is relatively common in some neurodegenerative proteinopathies of the brain and may be pathogenetically and diagnostically important. In Parkinson’s disease, neuronal α-synuclein aggregates are distributed throughout the nervous system, including the central nervous system (CNS), sympathetic ganglia, enteric nervous system, cardiac and pelvic plexuses, submandibular gland, adrenal medulla and skin. The pathological process may target the PNS and CNS at the same time. In multiple system atrophy, numerous glial cytoplasmic inclusions composed of filamentous α-synuclein are widely distributed in the CNS, while α-synuclein accumulation is minimal in the sympathetic ganglia and is restricted to neurons. Neurofibrillary tangles can occur in the sympathetic and spinal ganglia in tauopathy, although they appear to develop independently of cerebral Alzheimer’s disease pathology. In amyotrophic lateral sclerosis, neuronal loss with TDP-43-positive neuronal cytoplasmic inclusions in the spinal ganglia is more frequent than previously thought. Peripheral ganglia and visceral organs are also involved in polyglutamine diseases. Further elucidation and characterization of PNS lesions will have implications for intravital biopsy diagnosis in neurodegenerative proteinopathy, particularly in Parkinson’s disease.

Similar content being viewed by others
References
Abbott RD, Ross GW, Petrovitch H et al (2007) Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord 22:1581–1586
Aguzzi A (2009) Cell biology: beyond the prion principle. Nature 459:924–925
Altschuler E (1996) Gastric Helicobacter pylori infection as a cause of idiopathic Parkinson disease and non-arteric anterior optic ischemic neuropathy. Med Hypotheses 47:413–414
Amino T, Orimo S, Itoh Y, Takahashi A, Uchihara T, Mizusawa H (2005) Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol 15:29–34
Anderson G, Noorian AR, Taylor G et al (2007) Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp Neurol 207:4–12
Anlauf M, Schäfer MK, Eiden L, Weihe E (2003) Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. J Comp Neurol 459:90–111
Arai K, Kato N, Kawashima K, Hattori T (2000) Pure autonomic failure in association with human α-synucleinopathy. Neurosci Lett 296:171–173
Arai T, Hasegawa M, Akiyama H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Arima K, Uéda K, Sunohara N et al (1998) NACP/α-synuclein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol 96:439–444
Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S (2004) Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431:805–810
Beach TG, Adler CH, Sue LI et al (2008) Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol 115:445–451
Beach TG, Adler CH, Sue LI et al (2010) Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119:689–702
Blackett H, Walker R, Wood B (2009) Urinary dysfunction in Parkinson’s disease: a review. Parkinsonism Relat Disord 15:81–87
Bloch A, Probst A, Bissig H, Adams H, Tolnay M (2006) α-Synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 32:284–295
Bohl J, Ulbricht D, Steinmetz H (1997) Neurofibrillary tangles in peripheral autonomic ganglion cells. In: Iqbal K, Winblad B, Nishimura T, Takeda M, Wisniewski HM (eds) Alzheimer’s disease: biology. Diagnosis and therapeutics. Wiley, Chichester, pp 281–287
Braak H, Alafuzoff I, Arzberger T et al (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404
Braak H, Bohl JR, Müller CM et al (2006) The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21:2042–2051
Braak H, Braak E (1989) Cortical and subcortical argyrophilic grains characterize a disease associated with adult onset dementia. Neuropathol Appl Neurobiol 15:13–26
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, de Vos RAI, Bohl J, Del Tredici K (2006) Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396:67–72
Braak H, Del Tredici K (2008) Nervous system pathology in sporadic Parkinson’s disease. Neurology 70:1916–1925
Braak H, Del Tredici K, Rüb U et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Braak H, Rüb U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 110:517–536
Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K (2007) Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol 113:421–429
Braak H, Sastre M, Del Tredici K (2007) Development of α-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol 114:231–241
Brownell B, Oppenheimer DR, Hughes JT (1970) The central nervous system in motor neurone disease. J Neurol Neurosurg Psychiatry 33:338–357
Cersosimo MG, Benarroch EE (2008) Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord 23:1065–1075
Chaumette T, Lebouvier T, Aubert P et al (2009) Neurochemical plasticity in the enteric nervous system of a primate animal model of experimental Parkinsonism. Neurogastroenterol Motil 21:215–222
Croisier E, Graeber MB (2006) Glial degeneration and reactive gliosis in alpha-synucleinopathies: the emerging concept of primary gliodegeneration. Acta Neuropathol 112:517–530
Dabby R, Djaldetti R, Shahmurov M et al (2006) Skin biopsy for assessment of autonomic denervation in Parkinson’s disease. J Neural Transm 113:1169–1176
Dabir DV, Robinson MB, Swanson E et al (2006) Impaired glutamate transport in a mouse model of tau pathology in astrocytes. J Neurosci 26:644–654
Davidson Y, Kelley T, Mackenzie IR et al (2007) Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol 113:521–533
Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H (2010) Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol 119:703–707
Del Tredici K, Rüb U, De Vos RA, Bohl JR, Braak H (2002) Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61:413–426
DelleDonne A, Klos J, Fujishiro H et al (2008) Incidental Lewy body disease and preclinical Parkinson’s disease. Arch Neurol 65:1074–1080
Den Hartog Jager WA (1970) Histochemistry of adrenal bodies in Parkinson’s disease. Arch Neurol 23:528–533
Den Hartog Jager WA, Bethlem J (1960) The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry 23:283–290
Dickson DW, Bergeron C, Chin SS et al (2002) Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946
Dickson DW, Fujishiro H, DelleDonne A et al (2008) Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol 115:437–444
Dickson DW, Fujishiro H, Orr C et al (2009) Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord 15(Suppl 3):S1–S5
Djaldetti R, Lev N, Melamed E (2009) Lesions outside the CNS in Parkinson’s disease. Mov Disord 24:793–800
Dobbs RJ, Dobbs SM, Weller C et al (2008) Helicobacter hypothesis for idiopathic Parkinsonism: before and beyond. Helicobacter 13:309–322
Engelender S (2008) Ubiquitination of α-synuclein and autophagy in Parkinson’s disease. Autophagy 4:372–374
Ferrer I, Santpere G, van Leeuwen FW (2008) Argyrophilic grain disease. Brain 131:1416–1432
Forno LS, Norville RL (1976) Ultrastructure of Lewy bodies in the stellate ganglion. Acta Neuropathol 34:183–197
Foulds PG, Davidson Y, Mishra M et al (2009) Plasma phosphorylated-TDP-43 protein levels correlate with brain pathology in frontotemporal lobar degeneration. Acta Neuropathol 118:647–658
Foulds P, McAuley E, Gibbons L et al (2008) TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer’s disease and frontotemporal lobar degeneration. Acta Neuropathol 116:141–146
Fujishiro H, Frigerio R, Burnett M et al (2008) Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson’s disease. Mov Disord 23:1085–1092
Fujiwara H, Hasegawa M, Dohmae N et al (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nature Cell Biol 4:160–164
Fumimura Y, Ikemura M, Saito Y et al (2007) Analysis of the adrenal gland is useful for evaluating pathology of the peripheral autonomic nervous system in Lewy body disease. J Neuropathol Exp Neurol 66:354–362
Ghebremedhin E, Del Tredici K, Langston JW, Braak H (2009) Diminished tyrosine hydroxylase immunoreactivity in the cardiac conduction system and myocardium in Parkinson’s disease: an anatomical study. Acta Neuropathol 118:777–784
Hague K, Lento P, Morgello S, Caro S, Kaufmann H (1997) The distribution of Lewy bodies in pure autonomic failure: autopsy findings and review of the literature. Acta Neuropathol 94:192–196
Hasegawa M, Arai T, Nonaka T et al (2008) Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64:60–70
Hattori M, Hashizume Y, Yoshida M et al (2003) Distribution of astrocytic plaques in the corticobasal degeneration brain and comparison with tuft-shaped astrocytes in the progressive supranuclear palsy brain. Acta Neuropathol 106:143–149
Hawkes CH, Del Tredici K, Braak H (2007) Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33:599–614
Hayashi Y, Kakita A, Yamada M et al (1998) Hereditary dentatorubral-pallidoluysian atrophy: detection of widespread ubiquitinated neuronal and glial intranuclear inclusions in the brain. Acta Neuropathol 96:547–552
Herzog E (1928) Histopathologische Veränderungen im Sympathicus und ihre Bedeutung. Dtsch Z Nervenheilk 107:75–80
Higuchi M, Zhang B, Forman MS et al (2005) Axonal degeneration induced by targeted expression of mutant human tau in oligodendrocytes of transgenic mice that model glial tauopathies. J Neurosci 25:9434–9443
Horimoto Y, Matsumoto M, Akatsu H et al (2003) Autonomic dysfunctions in dementia with Lewy bodies. J Neurol 250:530–533
Ikeda K, Haga C, Akiyama H et al (1992) Coexistence of paired helical filaments and glial filaments in astrocytic processes within ghost tangles. Neurosci Lett 148:126–128
Ikemura M, Saito Y, Sengoku R et al (2008) Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol 67:945–953
Iwanaga K, Wakabayashi K, Yoshimoto M et al (1999) Lewy body-type degeneration in cardiac plexus in Parkinson’s and incidental Lewy body diseases. Neurology 52:1269–1271
Iwasaki Y, Yoshida M, Hashizume Y, Hattori M, Aiba I, Sobue G (2007) Widespread spinal cord involvement in progressive supranuclear palsy. Neuropathology 27:331–340
Iwasaki Y, Yoshida M, Hattori M et al (2004) Distribution of tuft-shaped astrocytes in the cerebral cortex in progressive supranuclear palsy. Acta Neuropathol 108:399–405
Iwasaki Y, Yoshida M, Hattori M, Hashizume Y, Sobue G (2005) Widespread spinal cord involvement in corticobasal degeneration. Acta Neuropathol 109:632–638
Jackson M, Lennox G, Balsitis M, Lowe J (1995) Lewy body dysphagia. J Neurol Neurosurg Psychiatry 58:756–758
Kanazawa M, Sanpei K, Toyoshima Y, Kawachi I, Honma Y, Takahashi H (2007) An autopsy case of dementia with Lewy bodies showing autonomic failure and dementia as the initial symptoms. Mov Disord 22:1212–1213
Kanda T, Tsukagoshi H, Oda M, Miyamoto K, Tanabe H (1996) Changes of unmyelinated nerve fibers in sural nerve in amyotrophic lateral sclerosis, Parkinson’s disease and multiple system atrophy. Acta Neuropathol 91:145–154
Kasai T, Tokuda T, Ishigami N et al (2009) Increased TDP-43 protein in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neuropathol 117:55–62
Kato T, Hirano A, Weinberg MN, Jacobs AK (1986) Spinal cord lesions in progressive supranuclear palsy: some new observations. Acta Neuropathol 71:11–14
Kaufmann H, Hague K, Perl D (2001) Accumulation of alpha-synuclein in autonomic nerves in pure autonomic failure. Neurology 56:980–981
Kaufmann H, Nahm K, Purohit D, Wolfe D (2004) Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology 63:1093–1095
Kawano H, Okada R, Yano K (2003) Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels 18:32–39
Kawasaki H, Murayama S, Tomonaga M, Izumiyama N, Shimada H (1987) Neurofibrillary tangles in human upper cervical ganglia: morphological study with immunohistochemistry and electron microscopy. Acta Neuropathol 75:156–159
Kikuchi H, Doh-ura K, Kira J, Iwaki T (1999) Preferential neurodegeneration in the cervical spinal cord of progressive supranuclear palsy. Acta Neuropathol 97:577–584
Klos KJ, Ahlskog JE, Josephs KA et al (2006) α-Synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology 66:1100–1102
Komori T (1999) Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol 9:663–679
Konagaya M, Sakai M, Matsuoka Y, Konagaya Y, Hashizume Y (1999) Multiple system atrophy with remarkable frontal atrophy. Acta Neuropathol 97:423–428
Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14:504–506
Kosaka K (1978) Lewy bodies in cerebral cortex. Report of three cases. Acta Neuropathol 42:127–134
Kosaka K, Iseki E, Odawara T, Yamamoto T (1996) Cerebral type of Lewy body disease. Neuropathology 16:32–35
Kovacs GG, Botond G, Budka H (2010) Protein coding of neurodegenerative dementias: the neuropathological basis of biomarker diagnostics. Acta Neuropathol 119:389–408
Kövari E, Burkhardt K, Lobrinus JA, Bouras C (2007) Lewy body dysphagia. Acta Neuropathol 114:295–298
Kuo YM, Li Z, Jiao Y, Gaborit N et al (2010) Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated α-synuclein gene mutations precede central nervous system changes. Hum Mol Genet 19:1633–1650
Kupsky WJ, Grimes MM, Sweeting J, Bertsch R, Cote LJ (1987) Parkinson’s disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology 37:1253–1255
Langston JW (2006) The Parkinson’s complex: Parkinsonism is just the tip of the iceberg. Ann Neurol 59:591–596
Lebouvier T, Chaumette T, Damier P et al (2008) Pathological lesions in colonic biopsies during Parkinson’s disease. Gut 57:1741–1743
Lerner A, Bagic A (2008) Olfactory pathogenesis of idiopathic Parkinson disease revisited. Mov Disord 23:1076–1084
Li JY, Englund E, Holton JL et al (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14:501–503
Li M, Nakagomi Y, Kobayashi Y et al (1998) Nonneural nuclear inclusions of androgen receptor protein in spinal and bulbar muscular atrophy. Am J Pathol 153:695–701
Lim SY, Fox SH, Lang AE (2009) Overview of the extranigral aspects of Parkinson disease. Arch Neurol 66:167–172
Manome T, Kobayashi T, Oguchi K, Tsukagoshi H, Fujiwara M (1980) An autopsy case of amyotrophic lateral sclerosis with dysesthesia and superficial sensory disturbance. Clin Neurol (Tokyo) 20:225–232
Matsumoto S, Hirano A, Goto S (1990) Spinal cord neurofibrillary tangles of Guamanian amyotrophic lateral sclerosis and parkinsonism-dementia complex: an immunohistochemical study. Neurology 40:975–979
Meco G, Rubino A, Caravona N, Valente M (2008) Sexual dysfunction in Parkinson’s disease. Parkinsonism Relat Disord 14:451–456
Miki Y, Mori F, Wakabayashi K, Kuroda N, Orimo S (2009) Incidental Lewy body disease restricted to the heart and stellate ganglia. Mov Disord 24:2299–2301
Miki Y, Tomiyama M, Ueno T et al (2010) Clinical availability of skin biopsy in the diagnosis of Parkinson’s disease. Neurosci Lett 469:357–359
Miller N, Noble E, Jones D, Burn D (2006) Hard to swallow: dysphagia in Parkinson’s disease. Age Ageing 35:614–618
Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F et al (2007) Do α-synuclein aggregates in autonomic plexuses predate Lewy body disorders? Neurology 68:2012–2018
Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K, Hirata K (2006) Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology 67:2236–2238
Mori F, Inenaga C, Yoshimoto M et al (2002) α-Synuclein immunoreactivity in normal and neoplastic Schwann cells. Acta Neuropathol 103:145–151
Mori F, Nishie M, Kakita A, Yoshimoto M, Takahashi H, Wakabayashi K (2006) Relationship among α-synuclein accumulation, dopamine synthesis, and neurodegeneration in Parkinson disease substantia nigra. J Neuropathol Exp Neurol 65:808–815
Mori F, Tanji K, Zhang H, Kakita A, Takahashi H, Wakabayashi K (2008) α-Synuclein pathology in the neostriatum in Parkinson’s disease. Acta Neuropathol 115:453–459
Mori F, Tanji K, Zhang HX et al (2008) Maturation process of TDP-43-positive neuronal cytoplasmic inclusions in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol 116:193–203
Nakano I, Iwatsubo T, Otsuka N et al (1992) Paired helical filaments in astrocytes: Electron microscopy and immunohistochemistry in a case of atypical Alzheimer’s disease. Acta Neuropathol 83:228–232
Nakazato Y, Yamazaki H, Hirato J, Ishida Y, Yamaguchi H (1990) Oligodendroglial microtubular tangles in multiple system atrophy. J Neuropathol Exp Neurol 49:521–530
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Nishie M, Mori F, Fujiwara H et al (2004) Accumulation of phosphorylated α-synuclein in the brain and peripheral ganglia of patients with multiple system atrophy. Acta Neuropathol 107:292–298
Nishie M, Mori F, Yoshimoto M, Takahashi H, Wakabayashi K (2004) A quantitative investigation of neuronal cytoplasmic and intranuclear inclusions in the pontine and inferior olivary nuclei in multiple system atrophy. Neuropathol Appl Neurobiol 30:546–554
Nishihira Y, Tan CF, Hoshi Y et al (2009) Sporadic amyotrophic lateral sclerosis of long duration is associated with relatively mild TDP-43 pathology. Acta Neuropathol 117:45–53
Nishihira Y, Tan CF, Onodera O et al (2008) Sporadic amyotrophic lateral sclerosis: two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathol 116:169–182
Nishihira Y, Tan CF, Toyoshima Y et al (2009) Sporadic amyotrophic lateral sclerosis: widespread multisystem degeneration with TDP-43 pathology in a patient after long-term survival on a respirator. Neuropathology 29:689–696
Nishimura M, Namba Y, Ikeda K, Akiguchi I, Oda M (1993) Neurofibrillary tangles in the neurons of spinal dorsal root ganglia of patients with progressive supranuclear palsy. Acta Neuropathol 85:453–457
Nolano M, Provitera V, Estraneo A et al (2008) Sensory deficit in Parkinson’s disease: evidence of a cutaneous denervation. Brain 131:1903–1911
Ohama E, Ikuta F (1976) Parkinson’s disease. Distribution of Lewy bodies and monoamine neurons system. Acta Neuropathol 34:311–319
Oinas M, Paetau A, Myllykangas L, Notkola IL, Kalimo H, Polvikoski T (2010) α-Synuclein pathology in the spinal cord autonomic nuclei associates with α-synuclein pathology in the brain: a population-based Vantaa 85+ study. Acta Neuropathol 119:715–722
Olanow CW, Prusiner SB (2009) Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci USA 106:12571–12572
Orimo S, Amino T, Itoh Y et al (2005) Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol 109:583–588
Orimo S, Amino T, Uchihara T et al (2007) Decreased cardiac uptake of MIBG is a potential biomarker for the presence of Lewy bodies. J Neurol 254(Suppl 4):21–28
Orimo S, Amino T, Yokochi M et al (2005) Preserved cardiac sympathetic nerve accounts for normal cardiac uptake of MIBG in PARK2. Mov Disord 20:1350–1353
Orimo S, Kanazawa T, Nakamura A et al (2007) Degeneration of cardiac sympathetic nerve can occur in multiple system atrophy. Acta Neuropathol 113:81–86
Orimo S, Oka T, Miura H et al (2002) Sympathetic cardiac denervation in Parkinson’s disease and pure autonomic failure but not in multiple system atrophy. J Neurol Neurosurg Psychiatry 73:776–777
Orimo S, Takahashi A, Uchihara T et al (2007) Degeneration of cardiac sympathetic nerve begins in the early disease process of Parkinson’s disease. Brain Pathol 17:24–30
Orimo S, Uchihara T, Nakamura A et al (2008) Axonal α-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 131:642–650
Orimo S, Uchihara T, Nakamura A et al (2008) Cardiac sympathetic denervation in Parkinson’s disease linked to SNCA duplication. Acta Neuropathol 116:575–577
Oyanagi K, Wakabayashi K, Ohama E et al (1990) Lewy bodies in the lower sacral parasympathetic neurons of a patient with Parkinson’s disease. Acta Neuropathol 80:558–559
Ozawa T, Paviour D, Quinn NP et al (2004) The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain 127:2657–2671
Papp MI, Kahn JE, Lantos PL (1989) Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 94:79–100
Parent A (1996) Carpenter’s human neuroanatomy, 9th edn. Williams & Wilkins, Baltimore
Pfeiffer RF (2003) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 2:107–116
Phillips RJ, Walter GC, Ringer BE, Higgs KM, Powley TL (2009) Alpha-synuclein immunopositive aggregates in the myenteric plexus of the aging Fischer 344 rat. Exp Neurol 220:109–119
Piao YS, Hayashi S, Hasegawa M et al (2001) Co-localization of α-synuclein and phosphorylated tau in neuronal and glial cytoplasmic inclusions in a patient with multiple system atrophy of long duration. Acta Neuropathol 101:285–293
Piao YS, Wakabayashi K, Kakita A et al (2003) Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol 13:10–22
Qualman SJ, Haupt HM, Yang P, Hamilton SR (1984) Esophageal Lewy bodies associated with ganglion cell loss in achalasia Similarity to Parkinson’s disease. Gastroenterology 87:848–856
Ross GW, Abbott RD, Petrovitch H et al (2006) Association of olfactory dysfunction with incidental Lewy bodies. Mov Disord 21:2062–2067
Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10(Suppl):S10–S17
Saito Y, Kawashima A, Ruberu NN et al (2003) Accumulation of phosphorylated α-synuclein in aging human brain. J Neuropathol Exp Neurol 62:644–654
Saito Y, Murayama S (2000) Expression of tau immunoreactivity in the spinal motor neurons of Alzheimer’s disease. Neurology 55:1727–1729
Saito Y, Rubbru NN, Sawabe M et al (2004) Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol 63:911–918
Sathasivam K, Hobbs C, Turmaine M et al (1999) Formation of polyglutamine inclusions in non-CNS tissue. Hum Mol Genet 8:813–822
Schmidt RE, Beaudet LN, Plurad SB, Dorsey DA (1997) Axonal cytoskeletal pathology in aged and diabetic human sympathetic autonomic ganglia. Brain Res 769:375–383
Sengoku R, Saito Y, Ikemura M et al (2008) Incidence and extent of Lewy body-related α-synucleinopathy in aging human olfactory bulb. J Neuropathol Exp Neurol 67:1072–1083
Shankle WR, Landing BH, Ang SM, Chui H, Villarreal-Engelhardt G, Zarow C (1993) Studies of the enteric nervous system in Alzheimer disease and other dementias of the elderly: enteric neurons in Alzheimer disease. Modern Pathol 6:10–14
Shibuya K, Nagatomo H, Iwabuchi K, Inoue M, Yagishita S, Itoh Y (2000) Asymmetrical temporal lobe atrophy with massive neuronal inclusions in multiple system atrophy. J Neurol Sci 179:50–58
Shishido T, Ikemura M, Obi T et al (2010) α-Synuclein accumulation in skin nerve fibers revealed by skin biopsy in pure autonomic failure. Neurology 74:608–610
Singaram C, Ashraf W, Gaumnitz EA et al (1995) Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 346:861–864
Sone M, Yoshida M, Hashizume Y, Hishikawa N, Sobue G (2005) α-Synuclein-immunoreactive structure formation is enhanced in sympathetic ganglia of patients with multiple system atrophy. Acta Neuropathol 110:19–26
Steele JC, Richardson JC, Olszewski J (1964) Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 10:333–359
Su M, Yoshida Y, Hirata Y, Watahiki Y, Nagata K (2001) Primary involvement of the motor area in association with the nigrostriatal pathway in multiple system atrophy: neuropathological and morphometric evaluations. Acta Neuropathol 101:57–64
Sugie M, Goto J, Kawamura M, Ota H (2005) Increased norepinephrine-associated adrenomedullary inclusions in Parkinson’s disease. Pathol Int 55:130–136
Suzuki M, Mikami H, Watanabe T et al (2010) Increased expression of TDP-43 in the skin of amyotrophic lateral sclerosis. Acta Neurol Scand (epub ahead of print). doi:10.1111/j.1600-0404.2010.01321.x
Takahashi H, Ohama E, Ikuta F (1991) Are Bunina bodies of endoplasmic reticulum origin? An ultrastructural study of subthalamic eosinophilic inclusions in a case of atypical motor neuron disease. Acta Pathol Jpn 41:889–894
Takahashi H, Oyanagi K, Takeda S, Hinokuma K, Ikuta F (1989) Occurrence of 15-nm-wide straight tubules in neocortical neurons in progressive supranuclear palsy. Acta Neuropathol 79:233–239
Takeda S, Yamazaki K, Miyakawa T, Arai H (1993) Parkinson’s disease with involvement of the parasympathetic ganglia. Acta Neuropathol 86:397–398
Takeda S, Yamazaki K, Miyakawa T, Arai H (1994) Morphological investigations of the peripheral autonomic nervous system in Parkinson’s disease, progressive supranuclear palsy and senile dementia of Alzheimer type. Brain Pathol 4:531
Taniwaki T, Minohara M, Hara H, Ohyagi Y, Yamada T, Kira J (2000) Striatonigral degeneration with motor neuron disease. J Neurol 247:395–396
Tashiro K, Moriwaka F, Matsuura T, Matsumoto A, Hamada T (1988) Sensory findings in amyotrophic lateral sclerosis. In: Tsubaki T, Yase Y (eds) Amytrophic lateral sclerosis. Elsevier, Amsterdam, pp 183–188
Tellez-Nagel I, Wisniewski HM (1973) Ultrastructure of neurofibrillary tangles in Steele-Richardson-Olszewski syndrome. Arch Neurol 29:324–327
Terry RD (2000) Do neuronal inclusions kill the cell? J Neural Transm (Suppl) 59:91–93
Tomonaga M, Saito M, Yoshimura M, Shimada H, Tohgi H (1978) Ultrastructure of the Bunina bodies in anterior horn cells of amyotrophic lateral sclerosis. Acta Neuropathol 42:81–86
Tsika E, Moysidou M, Guo J et al (2010) Distinct region-specific α-synuclein oligomers in A53T transgenic mice: Implications for neurodegeneration. J Neurosci 30:3409–3418
Tsuchiya K, Ozawa E, Haga C et al (2000) Constant involvement of the Betz cells and pyramidal tract in multiple system atrophy: a clinicopathological study of seven autopsy cases. Acta Neuropathol 99:628–636
van Ingelghem E, van Zandijcke M, Lammens M (1994) Pure autonomic failure: a new case with clinical, biochemical, and necropsy data. J Neurol Neurosurg Psychiatry 57:745–747
Wakabayashi K, Furuta A, Takahashi H, Ikuta F (1989) Occurrence of neurofibrillary tangles in the celiac ganglia. Acta Neuropathol 78:448
Wakabayashi K, Hayashi S, Kakita A et al (1998) Accumulation of α-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol 96:445–452
Wakabayashi K, Hayashi S, Morita T, Shibasaki Y, Watanabe Y, Takahashi H (1999) Neurofibrillary tangles in the peripheral sympathetic ganglia of non-Alzheimer elderly individuals. Clin Neuropathol 18:171–175
Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H (2000) NACP/α-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol 99:14–20
Wakabayashi K, Horikawa Y, Oyake M, Suzuki S, Morita T, Takahashi H (1998) Sporadic motor neuron disease with severe sensory neuronopathy. Acta Neuropathol 95:426–430
Wakabayashi K, Takahashi H (1997) The intermediolateral nucleus and Clarke’s column in Parkinson’s disease. Acta Neuropathol 94:287–289
Wakabayashi K, Takahashi H (1997) Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol 38(Suppl 2):2–7
Wakabayashi K, Takahashi H (2006) Cellular pathology in multiple system atrophy. Neuropathology 26:338–345
Wakabayashi K, Takahashi H, Obata K, Ikuta F (1992) Immunocytochemical localization of synaptic vesicle-specific protein in Lewy body-containing neurons in Parkinson’s disease. Neurosci Lett 138:237–240
Wakabayashi K, Takahashi H, Ohama E, Ikuta F (1989) Tyrosine hydroxylase-immunoreactive intrinsic neurons in the Auerbach’s and Meissner’s plexuses of humans. Neurosci Lett 96:259–263
Wakabayashi K, Takahashi H, Ohama E, Ikuta F (1990) Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol 79:581–583
Wakabayashi K, Takahashi H, Ohama E, Takeda S, Ikuta F (1993) Lewy bodies in the visceral autonomic nervous system in Parkinson’s disease. Adv Neurol 60:609–612
Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F (1988) Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76:217–221
Wakabayashi K, Tanji K, Mori F, Takahashi H (2007) The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of α-synuclein aggregates. Neuropathology 27:494–506
Wenning GK, Stefanova N, Jellinger KA, Poewe W, Schlossmacher MG (2008) Multiple system atrophy: a primary oligodendrogliopathy. Ann Neurol 64:239–246
Williams AJ, Paulson HL (2008) Polyglutamine neurodegeneration: protein misfolding revisited. Trends Neurosci 31:521–528
Yamada M, Hayashi S, Tsuji S, Takahashi H (2001) Involvement of the cerebral cortex and autonomic ganglia in Machado-Joseph disease. Acta Neuropathol 101:140–144
Yamada M, Sato T, Tsuji S, Takahashi H (2008) CAG repeat disorder models and human neuropathology: similarities and differences. Acta Neuropathol 115:71–86
Yamada M, Wood JD, Shimohata T et al (2001) Widespread occurrence of intranuclear atrophin-1 accumulation in the central nervous system neurons of patients with dentatorubral-pallidoluysian atrophy. Ann Neurol 49:14–23
Yamanaka Y, Asahina M, Hiraga A, Sakakibara R, Oka H, Hattori T (2007) Over 10 years of isolated autonomic failure preceding dementia and Parkinsonism in 2 patients with Lewy body disease. Mov Disord 22:595–597
Acknowledgments
This work has been supported by Grants-in-Aid 20300123 (K.W.), 20591361 (F.M.) and 20590335 (K.T.) for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, a Grant for Hirosaki University Institutional Research (K.W.), and a Grant-in-Aid for Studies on the Development of Diagnostic Technique and Therapies for Lewy Body Disease, the Ministry of Health, Labour and Welfare, Japan (K.W.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wakabayashi, K., Mori, F., Tanji, K. et al. Involvement of the peripheral nervous system in synucleinopathies, tauopathies and other neurodegenerative proteinopathies of the brain. Acta Neuropathol 120, 1–12 (2010). https://doi.org/10.1007/s00401-010-0706-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-010-0706-x