Abstract
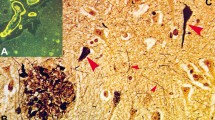
Alzheimer’s disease (AD) can be classified based on the relative density of neurofibrillary tangles (NFTs) in the hippocampus and association cortices into three subtypes: typical AD, hippocampal-sparing AD (HpSp AD), and limbic-predominant AD (LP AD). AD subtypes not only have pathologic, but also demographic, clinical, and genetic differences. Neurofibrillary tangle-predominant dementia (NFTD), a disorder with NFTs relatively restricted to limbic structures, shares this feature with LP AD raising the possibility that NFTD is a variant of AD. The objective criteria for pathologic diagnosis of NFTD are not available. A goal of this study was to design a mathematical algorithm that could diagnose NFTD from NFT and senile plaque (SP) counts in hippocampus and association cortices, analogous to that used to subtype AD. Moreover, we aimed to compare pathologic, demographic, clinical, and genetic features of NFTD (n = 18) with LP AD (n = 19), as well as the other AD subtypes, typical AD (n = 52) and HpSp AD (n = 17). Using digital microscopy, we confirmed that burden of phospho-tau (CP13) and of an NFT conformational epitope (Ab39) correlated with NFT densities and showed expected patterns across AD subtypes. HpSp AD had the highest and LP AD had the lowest burden of cortical CP13 and Ab39 immunoreactivity. On the other hand, cortical β-amyloid burden did not significantly differ between AD subtypes. Semi-quantitative assessment of SPs in the basal ganglia did show HpSp AD to have significantly more frequent presence of SPs compared to typical AD, which was more frequent than LP AD. Compared to LP AD, NFTD had an older age at disease onset and shorter disease duration, as well as lower Braak NFT stage. NFTs and SPs on thioflavin-S fluorescent microscopy, as well as CP13, Ab39, and Aβ immunoreactivities were very low in the frontal cortex of NFTD, differentiating NFTD from AD subtypes, including LP AD. MAPT H1H1 genotype frequency was high (~70 %) in NFTD and LP AD, and similar to typical AD, while APOE ε4 carrier state was low in NFTD. While it shares clinical similarities with regard to female sex predominance, onset in advanced age, and a slow cognitive decline, NFTD has significant pathologic differences from LP AD, suggesting that it may not merely be a variant of AD.




Similar content being viewed by others
References
Alladi S, Xuereb J, Bak T et al (2007) Focal cortical presentations of Alzheimer’s disease. Brain 130:2636–2645
Amador-Ortiz C, Lin WL, Ahmed Z et al (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445
Bancher C, Egensperger R, Kosel S, Jellinger K, Graeber MB (1997) Low prevalence of apolipoprotein E epsilon 4 allele in the neurofibrillary tangle predominant form of senile dementia. Acta Neuropathol 94:403–409
Barker WW, Luis CA, Kashuba A et al (2002) Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord 16:203–212
Braak H, Braak E (1990) Neurofibrillary changes confined to the entorhinal region and an abundance of cortical amyloid in cases of presenile and senile dementia. Acta Neuropathol 80:479–486
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259
Cairns N, Bigio E, Mackenzie I et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22
Dugger BN, Tu M, Murray ME, Dickson DW (2011) Disease specificity and pathologic progression of tau pathology in brainstem nuclei of Alzheimer’s disease and progressive supranuclear palsy. Neurosci Lett 491:122–126
Duyckaerts C, Delatour B, Potier M-C (2009) Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118:5–36
Espinoza M, de Silva R, Dickson DW, Davies P (2008) Differential incorporation of tau isoforms in Alzheimer’s disease. J Alzheimers Dis 14:1–16
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Galton CJ, Patterson K, Xuereb JH, Hodges JR (2000) Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 123(Pt 3):484–498
Hasegawa M, Arai T, Akiyama H et al (2007) TDP-43 is deposited in the Guam parkinsonism–dementia complex brains. Brain 130:1386–1394
Hatanpaa KJ, Bigio EH, Cairns NJ et al (2008) TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: a Midwest-Southwest Consortium for FTLD study. J Neuropathol Exp Neurol 67:271–279
Hyman BT, Trojanowski JQ (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 56:1095–1097
Hyman BT, West HL, Rebeck GW et al (1995) Quantitative analysis of senile plaques in Alzheimer disease: observation of log-normal size distribution and molecular epidemiology of differences associated with apolipoprotein E genotype and trisomy 21 (Down syndrome). Proc Natl Acad Sci USA 92:3586–3590
Ikeda K, Akiyama H, Arai T et al (1999) Clinical aspects of ‘senile dementia of the tangle type’— a subset of dementia in the senium separable from late-onset Alzheimer’s disease. Dement Geriatr Cogn Disord 10:6–11
Ikeda K, Akiyama H, Arai T et al (1997) A subset of senile dementia with high incidence of the apolipoprotein E epsilon2 allele. Ann Neurol 41:693–695
Iseki E, Yamamoto R, Murayama N et al (2006) Immunohistochemical investigation of neurofibrillary tangles and their tau isoforms in brains of limbic neurofibrillary tangle dementia. Neurosci Lett 405:29–33
Jellinger KA (2012) Neuropathological subtypes of Alzheimer’s disease. Acta Neuropathol 123:153–154
Jellinger KA, Attems J (2007) Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol 113:107–117
Jellinger KA, Bancher C (1998) Senile dementia with tangles (tangle predominant form of senile dementia). Brain Pathol 8:367–376
Josephs KA, Whitwell JL, Knopman DS et al (2008) Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 70:1850–1857
Khachaturian ZS (1985) Diagnosis of Alzheimer’s disease. Arch Neurol 42:1097–1105
Kim J, Miller VM, Levites Y et al (2008) BRI2 (ITM2b) inhibits Abeta deposition in vivo. J Neurosci 28:6030–6036
Knopman DS, Jack CR, Wiste HJ et al (2012) Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 78:1576–1582
Koivunen J, Verkkoniemi A, Aalto S et al (2008) PET amyloid ligand [11C]PIB uptake shows predominantly striatal increase in variant Alzheimer’s disease. Brain 131:1845–1853
Murray ME, Dickson DW (2008) O1-01-02: Alzheimer’s disease with relative hippocampal sparing: a distinct clinicopathologic variant. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 4:T106–1106
Murray ME, Graff-Radford NR, Ross OA et al (2012) Differentiating clinicopathologic and genetic aspects of hippocampal sclerosis in Alzheimer’s disease from limbic predominant Alzheimer’s disease and “pure” hippocampal sclerosis. Alzheimer’s Dementia J Alzheimer’s Assoc 8:P620–P621
Murray ME, Graff-Radford NR, Ross OA et al (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10:785–796
Nelson PT, Abner EL, Schmitt FA et al (2009) Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropathol Exp Neurol 68:774–784
Nitrini R, Lopes K, Brucki SMD (2011) Previous description of subtypes of Alzheimer’s pathology. Dement Neuropsychol 5:346–348
Noda K, Sasaki K, Fujimi K et al (2006) Quantitative analysis of neurofibrillary pathology in a general population to reappraise neuropathological criteria for senile dementia of the neurofibrillary tangle type (tangle-only dementia): the Hisayama Study. Neuropathology 26:508–518
Santa-Maria I, Haggiagi A, Liu X et al (2012) The MAPT H1 haplotype is associated with tangle-predominant dementia. Acta Neuropathol. doi:10.1007/s00401-012-1017-1
Schmechel DE, Saunders AM, Strittmatter WJ et al (1993) Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA 90:9649–9653
Terry RD, Hansen LA, DeTeresa R et al (1987) Senile dementia of the Alzheimer type without neocortical neurofibrillary tangles. J Neuropathol Exp Neurol 46:262–268
Togo T, Sahara N, Yen SH et al (2002) Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 61:547–556
Uchikado H, Lin WL, DeLucia MW, Dickson DW (2006) Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol 65:685–697
van der Flier WM, Pijnenburg YAL, Fox NC, Scheltens P (2011) Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE [var epsilon]4 allele. Lancet Neurology 10:280–288
van Duijn CM, de Knijff P, Cruts M et al (1994) Apolipoprotein E4 allele in a population-based study of early-onset Alzheimer’s disease. Nat Genet 7:74–78
Whitwell JL, Jack CR, Jr., Przybelski SA et al (2009) Temporoparietal atrophy: a marker of AD pathology independent of clinical diagnosis. Neurobiol Aging 32:1531–1541
Yen SH, Dickson DW, Crowe A, Butler M, Shelanski ML (1987) Alzheimer’s neurofibrillary tangles contain unique epitopes and epitopes in common with the heat-stable microtubule associated proteins tau and MAP2. Am J Pathol 126:81–91
Yokota O, Davidson Y, Bigio EH et al (2010) Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol 120:55–66
Yokota O, Terada S, Ishizu H et al (2002) NACP/alpha-synuclein immunoreactivity in diffuse neurofibrillary tangles with calcification (DNTC). Acta Neuropathol 104:333–341
Acknowledgments
The project was supported by the Mayo ADRC Grant (P50 AG16574), Mayo Clinic Study on Aging (U01 AG06786), Florida ADRC (P50 AG215711), Einstein Aging Study (P01 AG03949), Mangurian Foundation, and the State of Florida Alzheimer’s Disease Initiative. MEM and this project were supported by a fellowship from the Robert and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program and the Mayo ADRC Pilot Grant. OAR and DWD were supported by the Mayo Clinic Udall Center (P50 NS072187). OAR was partially supported by R01 NS078086. DWD was supported by the Robert E Jacoby Professorship for Alzheimer’s Research. We thank the patients and their families who donated brains to help further our knowledge in neurodegeneration. The authors would like to acknowledge the endless hours of commitment and teamwork offered by Linda G. Rousseau, Virginia R. Phillips, John Gonzalez, and Monica Castanedes-Casey. Without the sampling design and procedures for thioflavin-S fluorescent microscopy developed originally by Dr. Robert D. Terry [36], this study would not have been possible.
Ethical approval
All research reported is on postmortem material, which is considered exempt from human subject research. All brains were acquired with appropriate ethical approval and the research described has approval from the Mayo Clinic Institutional Review Board.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janocko, N.J., Brodersen, K.A., Soto-Ortolaza, A.I. et al. Neuropathologically defined subtypes of Alzheimer’s disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol 124, 681–692 (2012). https://doi.org/10.1007/s00401-012-1044-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-012-1044-y