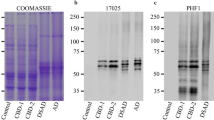
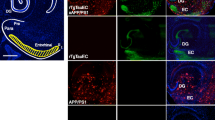
Abstract
Filamentous tau inclusions are hallmarks of Alzheimer’s disease (AD) and other neurodegenerative tauopathies. An increasing number of studies implicate the cell-to-cell propagation of tau pathology in the progression of tauopathies. We recently showed (Iba et al., J Neurosci 33:1024–1037, 2013) that inoculation of preformed synthetic tau fibrils (tau PFFs) into the hippocampus of young transgenic (Tg) mice (PS19) overexpressing human P301S mutant tau induced robust tau pathology in anatomically connected brain regions including the locus coeruleus (LC). Since Braak and colleagues hypothesized that the LC is the first brain structure to develop tau lesions and since LC has widespread connections throughout the CNS, LC neurons could be the critical initiators of the stereotypical spreading of tau pathology through connectome-dependent transmission of pathological tau in AD. Here, we report that injections of tau PFFs into the LC of PS19 mice induced propagation of tau pathology to major afferents and efferents of the LC. Notably, tau pathology propagated along LC efferent projections was localized not only to axon terminals but also to neuronal perikarya, suggesting transneuronal transfer of templated tau pathology to neurons receiving LC projections. Further, brainstem neurons giving rise to major LC afferents also developed perikaryal tau pathology. Surprisingly, while tangle-bearing neurons degenerated in the LC ipsilateral to the injection site starting 6 months post-injection, no neuron loss was seen in the contralateral LC wherein tangle-bearing neurons gradually cleared tau pathology by 6–12 months post-injection. However, the spreading pattern of tau pathology observed in our LC-injected mice is different from that in AD brains since hippocampus and entorhinal cortex, which are affected in early stages of AD, were largely spared of tau inclusions in our model. Thus, while our study tested critical aspects of the Braak hypothesis of tau pathology spread, this novel mouse model provides unique opportunities to elucidate mechanisms underlying the selective vulnerability of neurons to acquire tau pathology and succumb to or resist tau-mediated neurodegeneration.







Similar content being viewed by others
References
Ahmed Z, Cooper J, Murray TK, Garn K, McNaughton E, Clarke H, Parhizkar S, Ward MA, Cavallini A, Jackson S et al (2014) A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol 127:667–683. doi:10.1007/s00401-014-1254-6
Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42:631–639
Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450. doi:10.1146/annurev.neuro.28.061604.135709
Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT (1986) The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science 234:734–737
Ballatore C, Lee VM, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8:663–672. doi:10.1038/nrn2194
Berridge CW, Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev 42:33–84. doi:10.1016/S0165-0173(03)00143-7
Bobinski M, Wegiel J, Tarnawski M, de Leon MJ, Reisberg B, Miller DC, Wisniewski HM (1998) Duration of neurofibrillary changes in the hippocampal pyramidal neurons. Brain Res 799:156–158 (pii:S0006-8993(98)00441-7)
Boluda S, Iba M, Zhang B, Raible KM, Lee VM, Trojanowski JQ (2015) Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol 129:221–237. doi:10.1007/s00401-014-1373-0
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Del Tredici K (2011) The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121:171–181. doi:10.1007/s00401-010-0789-4
Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70:960–969. doi:10.1097/NEN.0b013e318232a379
Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ (2015) Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci 16:109–120. doi:10.1038/nrn3887
Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L (2010) Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci 13:1526–1533. doi:10.1038/nn.2682
Cedarbaum JM, Aghajanian GK (1978) Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol 178:1–16. doi:10.1002/cne.901780102
Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M et al (2013) Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci USA 110:9535–9540. doi:10.1073/pnas.1301175110
Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M et al (2009) Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11:909–913. doi:10.1038/ncb1901
Clavier RM (1979) Afferent projections to the self-stimulation regions of the dorsal pons, including the locus coeruleus, in the rat as demonstrated by the horseradish peroxidase technique. Brain Res Bull 4:497–504
de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA et al (2012) Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73:685–697. doi:10.1016/j.neuron.2011.11.033
Foote SL, Bloom FE, Aston-Jones G (1983) Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63:844–914
Frost B, Jacks RL, Diamond MI (2009) Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284:12845–12852. doi:10.1074/jbc.M808759200
Guo JL, Lee VM (2014) Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med 20:130–138. doi:10.1038/nm.3457
Guo JL, Lee VM (2013) Neurofibrillary tangle-like tau pathology induced by synthetic tau fibrils in primary neurons over-expressing mutant tau. FEBS Lett 587:717–723. doi:10.1016/j.febslet.2013.01.051
Guo JL, Lee VM (2011) Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem 286:15317–15331. doi:10.1074/jbc.M110.209296
Hurtado DE, Molina-Porcel L, Iba M, Aboagye AK, Paul SM, Trojanowski JQ, Lee VM (2010) Aβ accelerates the spatiotemporal progression of tau pathology and augments tau amyloidosis in an Alzheimer mouse model. Am J Pathol 177:1977–1988. doi:10.2353/ajpath.2010.100346
Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM (2013) Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J Neurosci 33:1024–1037. doi:10.1523/JNEUROSCI.2642-12.2013
Irwin DJ, Cairns NJ, Grossman M, McMillan CT, Lee EB, Van Deerlin VM, Lee VM, Trojanowski JQ (2015) Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol 129:469–491. doi:10.1007/s00401-014-1380-1
Kenessey A, Yen SH, Liu WK, Yang XR, Dunlop DS (1995) Detection of d-aspartate in tau proteins associated with Alzheimer paired helical filaments. Brain Res 675:183–189 (pii:0006-8993(95)00061-T)
Kuchibhotla KV, Wegmann S, Kopeikina KJ, Hawkes J, Rudinskiy N, Andermann ML, Spires-Jones TL, Bacskai BJ, Hyman BT (2014) Neurofibrillary tangle-bearing neurons are functionally integrated in cortical circuits in vivo. Proc Natl Acad Sci USA 111:510–514. doi:10.1073/pnas.1318807111
Lee VM, Goedert M, Trojanowski JQ (2001) Neurodegenerative tauopathies. Annu Rev Neurosci 24:1121–1159. doi:10.1146/annurev.neuro.24.1.1121
Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K (2012) Trans-synaptic spread of tau pathology in vivo. PLoS One 7:e31302. doi:10.1371/journal.pone.0031302
Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M (1995) Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience 65:119–160
Moore RY, Bloom FE (1979) Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci 2:113–168. doi:10.1146/annurev.ne.02.030179.000553
Morgane PJ, Jacobs MS (1979) Raphe projections to the locus coeruleus in the rat. Brain Res Bull 4:519–534
Morozova OA, March ZM, Robinson AS, Colby DW (2013) Conformational features of tau fibrils from Alzheimer’s disease brain are faithfully propagated by unmodified recombinant protein. Biochemistry 52:6960–6967. doi:10.1021/bi400866w
Morsch R, Simon W, Coleman PD (1999) Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol 58:188–197
Paxinos G, Franklin K (2003) The mouse brain in stereotaxic coordinates, 2nd edn. Academic, Waltham
Peeraer E, Bottelbergs A, Van Kolen K, Stancu IC, Vasconcelos B, Mahieu M, Duytschaever H, Ver Donck L, Torremans A, Sluydts E et al (2015) Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of tau transgenic mice. Neurobiol Dis 73:83–95. doi:10.1016/j.nbd.2014.08.032
Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E et al (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309:476–481. doi:10.1126/science.1113694
Stancu IC, Vasconcelos B, Ris L, Wang P, Villers A, Peeraer E, Buist A, Terwel D, Baatsen P, Oyelami T et al (2015) Templated misfolding of Tau by prion-like seeding along neuronal connections impairs neuronal network function and associated behavioral outcomes in Tau transgenic mice. Acta Neuropathol 129:875–894. doi:10.1007/s00401-015-1413-4
Van der Jeugd A, Hochgrafe K, Ahmed T, Decker JM, Sydow A, Hofmann A, Wu D, Messing L, Balschun D, D’Hooge R et al (2012) Cognitive defects are reversible in inducible mice expressing pro-aggregant full-length human Tau. Acta Neuropathol 123:787–805. doi:10.1007/s00401-012-0987-3
Wilcock GK, Esiri MM (1982) Plaques, tangles and dementia. A quantitative study. J Neurol Sci 56:343–356
Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB (2001) Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293:711–714. doi:10.1126/science.1062382
Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53:337–351. doi:10.1016/j.neuron.2007.01.010
Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, Hogan AM, Xie SX, Ballatore C, Smith AB 3rd et al (2012) The microtubule-stabilizing agent, epothilone d, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci 32:3601–3611. doi:10.1523/JNEUROSCI.4922-11.2012
Acknowledgments
This work was supported by National Institutes of Health Grant AG17586 and the Marian S. Ware Alzheimer Program Cure Alzheimer’s Fund, the Karen Cohen Segal and Christopher S. Segal Alzheimer Drug Discovery Initiative Fund, the Paula C. Schmerler Fund for Alzheimer’s Research, the Barrist Neurodegenerative Disease Research Fund, the Eleanor Margaret Kurtz Endowed Fund, the Mary Rasmus Endowed Fund for Alzheimer’s Research, Gloria J. Miller, and Dr. Arthur Peck. We thank Joshua Daniels, Bryan Zoll and Susan Leight for their assistance in mouse husbandry, Martine White and Chris Chung for counting cell numbers, Theresa Schuck, John Robinson, and Kevin Raible for their technical assistance in immunohistochemistry, and Rui Tong for his computer expertise in constructing the heat maps.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animal were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iba, M., McBride, J.D., Guo, J.L. et al. Tau pathology spread in PS19 tau transgenic mice following locus coeruleus (LC) injections of synthetic tau fibrils is determined by the LC’s afferent and efferent connections. Acta Neuropathol 130, 349–362 (2015). https://doi.org/10.1007/s00401-015-1458-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-015-1458-4