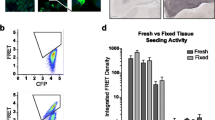
Abstract
Transcellular propagation of tau aggregates may underlie the progression of pathology in Alzheimer’s disease (AD) and other tauopathies. Braak staging (B1, B2, B3) is based on phospho-tau accumulation within connected brain regions: entorhinal cortex (B1); hippocampus/limbic system (B2); and frontal and parietal lobes (B3). We previously developed a specific and sensitive assay that uses flow cytometry to quantify tissue seeding activity based on fluorescence resonance energy transfer (FRET) in cells that stably express tau reporter proteins. In a tauopathy mouse model, we have detected seeding activity far in advance of histopathological changes. It remains unknown whether individuals with AD also develop seeding activity prior to accumulation of phospho-tau. We measured tau seeding activity across four brain regions (hippocampus, frontal lobe, parietal lobe, and cerebellum) in 104 fresh-frozen human AD brain samples from all Braak stages. We observed widespread seeding activity, notably in regions predicted to be free of phospho-tau deposition, and in detergent-insoluble fractions that lacked tau detectable by ELISA. Seeding activity correlated positively with Braak stage and negatively with MMSE. Our results are consistent with early transcellular propagation of tau seeds that triggers subsequent development of neuropathology. The FRET-based seeding assay may also complement standard neuropathological classification of tauopathies.




Similar content being viewed by others
References
Acker CM, Forest SK, Zinkowski R, Davies P, d’Abramo C (2013) Sensitive quantitative assays for tau and phospho-tau in transgenic mouse models. Neurobiol Aging 34:338–350. doi:10.1016/j.neurobiolaging.2012.05.010
Ahmed Z, Cooper J, Murray TK, Garn K, McNaughton E, Clarke H, Parhizkar S, Ward MA, Cavallini A, Jackson S et al (2014) A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol 127:667–683. doi:10.1007/s00401-014-1254-6
Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42:631–639
Boluda S, Iba M, Zhang B, Raible KM, Lee VM, Trojanowski JQ (2015) Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol 129:221–237. doi:10.1007/s00401-014-1373-0
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. doi:10.1007/s00401-006-0127-z
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–278 (discussion 278–284)
Calafate S, Buist A, Miskiewicz K, Vijayan V, Daneels G, de Strooper B, de Wit J, Verstreken P, Moechars D (2015) Synaptic contacts enhance cell-to-cell tau pathology propagation. Cell Rep 11:1176–1183. doi:10.1016/j.celrep.2015.04.043
Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckner N, Hanmer J, Davies P, O’Neill MJ et al (2011) Passive immunization with anti-tau antibodies in two transgenic models: reduction of tau pathology and delay of disease progression. J Biol Chem 286:34457–34467. doi:10.1074/jbc.M111.229633
Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M et al (2013) Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci USA 110:9535–9540. doi:10.1073/pnas.1301175110
Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M et al (2009) Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11:909–913. doi:10.1038/ncb1901
de Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz David H, Kopeikina Kathy J, Pitstick R, Sahara N, Ashe Karen H, Carlson George A et al (2012) Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73:685–697
Fritschi SK, Cintron A, Ye L, Mahler J, Buhler A, Baumann F, Neumann M, Nilsson KP, Hammarstrom P, Walker LC et al (2014) Abeta seeds resist inactivation by formaldehyde. Acta Neuropathol 128:477–484. doi:10.1007/s00401-014-1339-2
Frost B, Diamond MI (2009) Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci 11:155–159
Frost B, Jacks RL, Diamond MI (2009) Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284:12845–12852
Furman JL, Holmes BB, Diamond MI (2015) Sensitive detection of proteopathic seeding activity with FRET flow cytometry. J Vis Exp JoVE. doi:10.3791/53205
Grober E, Dickson D, Sliwinski MJ, Buschke H, Katz M, Crystal H, Lipton RB (1999) Memory and mental status correlates of modified Braak staging. Neurobiol Aging 20:573–579
Guo JL, Lee VMY (2011) Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem. doi:10.1074/jbc.M110.209296
Holmes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, Brodsky FM, Marasa J, Bagchi DP et al (2013) Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci USA 110:E3138–E3147. doi:10.1073/pnas.1301440110
Holmes BB, Furman JL, Mahan TE, Yamasaki TR, Mirbaha H, Eades WC, Belaygorod L, Cairns NJ, Holtzman DM, Diamond MI (2014) Proteopathic tau seeding predicts tauopathy in vivo. Proc Natl Acad Sci USA. doi:10.1073/pnas.1411649111
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8:1–13. doi:10.1016/j.jalz.2011.10.007
Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM (2013) Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J Neurosci 33:1024–1037. doi:10.1523/JNEUROSCI.2642-12.2013
Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI (2012) Trans-cellular propagation of tau aggregation by fibrillar species. J Biol Chem 287:19440–19451. doi:10.1074/jbc.M112.346072
Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K (2012) Trans-synaptic spread of tau pathology in vivo. PLoS One 7:e31302. doi:10.1371/journal.pone.0031302
Mirbaha H, Holmes BB, Sanders DW, Bieschke J, Diamond MI (2015) Tau trimers are the minimal propagation unit spontaneously internalized to seed intracellular aggregation. J Biol Chem 290:14893–14903. doi:10.1074/jbc.M115.652693
Peeraer E, Bottelbergs A, Van Kolen K, Stancu IC, Vasconcelos B, Mahieu M, Duytschaever H, Ver Donck L, Torremans A, Sluydts E et al (2015) Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of tau transgenic mice. Neurobiol Dis 73:83–95. doi:10.1016/j.nbd.2014.08.032
Polydoro M, Acker CM, Duff K, Castillo PE, Davies P (2009) Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J Neurosci 29:10741–10749. doi:10.1523/JNEUROSCI.1065-09.2009
Prusiner SB (1984) Some speculations about prions, amyloid, and Alzheimer’s disease. N Engl J Med 310:661–663. doi:10.1056/NEJM198403083101021
Raj A, Kuceyeski A, Weiner M (2012) A network diffusion model of disease progression in dementia. Neuron 73:1204–1215
Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li A, Barker SJ, Foley AC, Thorpe JR, Serpell LC et al (2014) Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. doi:10.1016/j.neuron.2014.04.047
Sanders DW, Kaufman SK, Holmes BB, Diamond MI (2016) Prions and protein assemblies that convey biological information in health and disease. Neuron 89:433–448. doi:10.1016/j.neuron.2016.01.026
Schweighauser M, Bacioglu M, Fritschi SK, Shimshek DR, Kahle PJ, Eisele YS, Jucker M (2015) Formaldehyde-fixed brain tissue from spontaneously ill alpha-synuclein transgenic mice induces fatal alpha-synucleinopathy in transgenic hosts. Acta Neuropathol 129:157–159. doi:10.1007/s00401-014-1360-5
Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD (2009) Neurodegenerative diseases target large-scale human brain networks. Neuron 62:42–52. doi:10.1016/j.neuron.2009.03.024
Small SA, Duff K (2008) Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron 60:534–542. doi:10.1016/j.neuron.2008.11.007
Stancu IC, Vasconcelos B, Ris L, Wang P, Villers A, Peeraer E, Buist A, Terwel D, Baatsen P, Oyelami T et al (2015) Templated misfolding of tau by prion-like seeding along neuronal connections impairs neuronal network function and associated behavioral outcomes in tau transgenic mice. Acta Neuropathol 129:875–894. doi:10.1007/s00401-015-1413-4
Weaver CL, Espinoza M, Kress Y, Davies P (2000) Conformational change as one of the earliest alterations of tau in Alzheimer’s disease. Neurobiol Aging 21:719–727
Yanamandra K, Kfoury N, Jiang H, Mahan TE, Ma S, Maloney SE, Wozniak DF, Diamond MI, Holtzman DM (2013) Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 80:402–414. doi:10.1016/j.neuron.2013.07.046
Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53:337–351. doi:10.1016/j.neuron.2007.01.010
Zhou J, Gennatas Efstathios D, Kramer Joel H, Miller Bruce L, Seeley William W (2012) Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 73:1216–1227
Acknowledgements
We thank Peter Davies for generously providing antibody reagents and ELISA protocol guidance. We thank the University of Texas, Southwestern Medical Center Alzheimer Disease Center, Washington University in St. Louis, the Sanders-Brown Center on Aging at the University of Kentucky, and Dr. Carol Tamminga in the Psychiatry Department at UT Southwestern Medical Center for providing pathological samples and corresponding clinical data. Ping Shang, HT(ASCP)QIHC, performed the immunohistochemical staining on tissue sections used in the Supp. Figure 1 illustrations, and Chan Foong, M.S., prepared the whole slide scanning images. Studies were supported by the Tau Consortium (to M.I.D), Coins for Alzheimer’s Research Trust (to N.J.C), and NIH grants awarded to M.I.D. (R01AG048678), J.L.F. (1F32NS087805), C.L.W. (AG12300), N.J.C. (P50 AG005681 and P01 AG003991) and P.T.N. (AG028383).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2016_1644_MOESM1_ESM.pdf
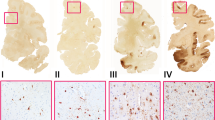
Supplemental Figure 1. Representative AT8 Staining of B1, B2, and B3 subjects. Hippocampus, frontal lobe, parietal lobe, and cerebellum stained with AT8 from representative subjects diagnosed as B1 (a-d), B2 (e-h), or B3 (i-l). Note that positive signal is restricted to entorhinal cortex (EC) in B1, spreads to hippocampus in B2, and to cortical regions in B3. Scale bar, 3 mm
401_2016_1644_MOESM2_ESM.pdf
Supplemental Figure 2. Representative cells with and without inclusions after transduction by tau seeds or control. Tau-CFP/YFP cells were transduced with empty liposomes (a), total (b, d, f, h, j) or insoluble (c, e, g, i, k) fractions. Variable degrees of aggregation were detectable with confocal microscopy
Rights and permissions
About this article
Cite this article
Furman, J.L., Vaquer-Alicea, J., White, C.L. et al. Widespread tau seeding activity at early Braak stages. Acta Neuropathol 133, 91–100 (2017). https://doi.org/10.1007/s00401-016-1644-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-016-1644-z