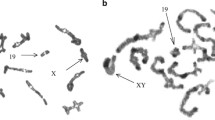
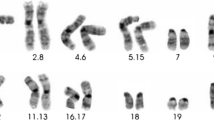
Abstract
Many different chromosomal races with reduced chromosome number due to the presence of Robertsonian fusion metacentrics have been described in western Europe and northern Africa, within the distribution area of the western house mouse Mus musculus domesticus. This subspecies of house mouse has become the ideal model for studies to elucidate the processes of chromosome mutation and fixation that lead to the formation of chromosomal races and for studies on the impact of chromosome heterozygosities on reproductive isolation and speciation. In this review, we briefly describe the history of the discovery of the first and subsequent metacentric races in house mice; then, we focus on the molecular composition of the centromeric regions involved in chromosome fusion to examine the molecular characteristics that may explain the great variability of the karyotype that house mice show. The influence that metacentrics exert on the nuclear architecture of the male meiocytes and the consequences on meiotic progression are described to illustrate the impact that chromosomal heterozygosities exert on fertility of house mice—of relevance to reproductive isolation and speciation. The evolutionary significance of the Robertsonian phenomenon in the house mouse is discussed in the final section of this review.





Similar content being viewed by others
References
Adega F, Guedes-Pinto H, Chaves R (2009) Satellite DNA in the karyotype evolution of domestic animals—clinical considerations. Cytogenet Genome Res 126:12–20
Adolph S, Klein J (1981) Robertsonian variation in Mus musculus from central Europe, Spain, and Scotland. J Heredity 72:219–221
Ashley T, Gaeth AP, Inagaki H, Seftel A, Cohen MM, Anderson LK, Kurahashi H, Emanuel BS (2006) Meiotic recombination and spatial proximity in the etiology of the recurrent t(11;22). Am J Hum Genet 79:524–538
Baarends WM, Wassenaar E, van der Laan R, Hoogerbrugge J, Sleddens-Linkels E, Hoeijmakers JH, de Boer P, Grootegoed JA (2005) Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol Cell Biol 25:1041–1053
Bandyopadhyay R, Berend SA, Page SL, Choo KH, Shaffer LG (2001) Satellite III sequences on 14p and their relevance to Robertsonian translocation formation. Chromosome Res 9:235–242
Basu J, Stromberg G, Compitello G, Willard HF, Van Bokkelen G (2005) Rapid creation of BAC-based human artificial chromosome vectors by transposition with synthetic alpha-satellite arrays. Nucleic Acids Res 33:587–596
Belkhir K, Bolomier V, Bonhomme F, Boursot P, Britton-Davidian J, Desmarais E, Catalan J, Catzeflis F, Orth A, Vanlerberghe F (1987) New Robertsonian population. Mouse News Letter 78:60
Berrios S, Manterola M, Prieto Z, Lopez-Fenner J, Page J, Fernandez-Donoso R (2010) Model of chromosome associations in Mus domesticus spermatocytes. Biol Res 43:275–285
Berríos S, Manieu C, López-Fenner J, Ayarza E, Page J, González M, Manterola M, Fernández-Donoso R (2014) Robertsonian chromosomes and the nuclear architecture of mouse meiotic prophase spermatocytes. Biol Res 47:16
Bonhomme F, Britton-Davidian J, Catalan J, Dabonneville F, Thaler L (1983) Robertsonian variation in the Balearic Isles. Mouse News Letter 69:35
Boursot P, Auffray J-C, Britton-Davidian J, Bonhomme F (1993) The evolution of house mice. Ann Rev Ecol Syst 24:119–152
Branco MR, Pombo A (2006) Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol 4:e138
Britton-Davidian J, Nadeau JH, Croset H, Thaler L (1989) Genetic differentiation and origin of Robertsonian populations of the house mouse (Mus musculus domesticus Rutty). Genet Res 53:29–44
Britton-Davidian J, Catalan J, Ramalhinho MG, Ganem G, Auffray J-C, Capela R, Biscoito M, Searle JB, Mathias MD (2000) Rapid chromosomal evolution in island mice. Nature 403:158
Britton-Davidian J, Catalan J, Ramalhinho MG, Auffray J-C, Nunes AC, Gazave E, Searle JB, Mathias ML (2005) Chromosomal phylogeny of Robertsonian races of the house mouse on the island of Madeira: testing between alternative mutational processes. Genet Res 86:171–183
Bryden W (1933) A comparison of the chromosomes of the rat and the mouse. J Genet 27:421–433
Burgoyne PS, Mahadevaiah SK, Turner JM (2009) The consequences of asynapsis for mammalian meiosis. Nat Rev Genet 10:207–216
Campbell P, Good JM, Nachman MW (2013) Meiotic sex chromosome inactivation is disrupted in sterile hybrid male house mice. Genetics 193:819–828
Capanna E (1982) Robertsonian numerical variation in animal speciation: Mus musculus, an emblematic model. In: Barigozzi C (ed) Mechanisms of speciation. Liss, New York, pp 155–177
Capanna E, Redi CA (1995) Whole-arm reciprocal translocation (WART) between Robertsonian chromosomes: finding of a Robertsonian heterozygous mouse with karyotype derived through WARTs. Chromosome Res 3:135–137
Capanna E, Gropp A, Winking H, Noack G, Civitelli MV (1976) Robertsonian metacentrics in the mouse. Chromosoma 58:341–353
Capanna E, Castiglia R, Solano E (2009) Men and mice: mouse population genetics in the Aeolian archipelago. In: Casellato S, Burighel P, Minelli A (eds) Life and time: the evolution of life and its history. Padova, Cleup, pp 59–74
Castiglia R, Capanna E (2000) Contact zone between chromosomal races of Mus musculus domesticus. 2. Fertility and segregation in laboratory-reared and wild mice heterozygous for multiple Robertsonian rearrangements. Heredity 85:147–156
Cazaux B, Catalan J, Justy F, Escudé C, Desmarais E, Britton-Davidian J (2013) Evolution of the structure and composition of house mouse satellite DNA sequences in the subgenus Mus (Rodentia: Muridea): a cytogenomic approach. Chromosoma 122:209–220
Cerda MC, Berríos S, Fernández-Donoso R, Garagna S, Redi CA (1999) Organization of complex nuclear domains in somatic mouse cells. Biol Cell 91:55–65
Chatti N, Ganem G, Benzekri K, Catalan J, Britton-Davidian J, Saïd K (1999) Microgeographical distribution of two chromosomal races of house mice in Tunisia: pattern and origin of habitat partitioning. Proc R Soc Lond B 266:1561–1569
Cucchi T, Vigne JD, Auffray J-C (2005) First occurrence of the house mouse (Mus musculus domesticus Schwarz & Schwarz, 1943) in the western Mediterranean: a zooarchaeological revision of sub-fossil house mouse occurrences. Biol J Linn Soc 84:429–445
De Rop V, Padeganeh A, Maddox PS (2012) CENP-A: the key player behind centromere identity, propagation, and kinetochore assembly. Chromosoma 121:527–538
Eaker S, Pyle A, Cobb J, Handel MA (2001) Evidence for meiotic spindle checkpoint from analysis of spermatocytes from Robertsonian-chromosome heterozygous mice. J Cell Sci 114:2953–2965
Everett CA, Searle JB, Wallace BM (1996) A study of meiotic pairing, nondisjunction and germ cell death in laboratory mice carrying Robertsonian translocations. Genet Res 67:239–247
Fachinetti D, Folco HD, Nechemia-Arbely Y, Valente LP, Nguyen K, Wong AJ, Zhu Q, Holland AJ, Desai A, Jansen LE, Cleveland DW (2013) A two-step mechanism for epigenetic specification of centromere identity and function. Nat Cell Biol 15:1056–1066
Fernández-Donoso R, Berríos S, Page J, Merani MS, Lizarralde MS, Vidal-Roja L, Bianchi NO (2001) Robertsonian chromosome polymorphism of Akodon molinae (Rodentia: Sigmodontidae): analysis of trivalents in meiotic prophase. Rev Chil Hist Nat 74:107–119
Franchini P, Castiglia R, Capanna E (2008) Reproductive isolation between chromosomal races of the house mouse Mus musculus domesticus in a parapatric contact area revealed by an analysis of multiple unlinked loci. J Evol Biol 21:502–513
Franchini P, Colangelo P, Solano E, Capanna E, Verheyen E, Castiglia R (2010) Reduced gene flow at pericentromeric loci in a hybrid zone involving chromosomal races of the house mouse Mus musculus domesticus. Evolution 64:2020–2032
Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, Beilharz EJ, Gupta RV, Montgomery J, Morenzoni MM, Nilsen GB, Pethiyagoda CL, Stuve LL, Johnson FM, Daly MJ, Wade CM, Cox DR (2007) A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature 448:1050–1053
Ganem G (2012) Behaviour, ecology, and speciation in the house mouse. In: Macholán M, Baird SJE, Munclinger P, Piálek J (eds) Evolution of the house mouse. Cambridge University Press, Cambridge, pp 373–406 (Cambridge Series in Morphology and Molecules)
Garagna S, Zuccotti M, Searle JB, Redi CA, Wilkinson PJ (1989) Spermatogenesis in heterozygotes for Robertsonian chromosomal rearrangements from natural populations of the common shrew, Sorex araneus. J Reprod Fertil 87:431–438
Garagna S, Redi CA, Zuccotti M, Britton-Davidian J, Winking H (1990) Kinetics of oogenesis in mice heterozygous for Robertsonian translocations. Differentiation 42:167–171
Garagna S, Redi CA, Capanna E, Andayani N, Alfano RM, Doi P, Viale G (1993) Genome distribution, chromosomal allocation, and organization of the major and minor satellite DNAs in 11 species and subspecies of the genus Mus. Cytogenet Cell Genet 64:247–255
Garagna S, Broccoli D, Redi CA, Searle JB, Cook HJ, Capanna E (1995) Robertsonian metacentrics of the house mouse lose telomeric sequences but retain some minor satellite DNA in the pericentromeric area. Chromosoma 103:685–692
Garagna S, Zuccotti M, Redi CA, Capanna E (1997) Trapping speciation. Nature 390:241–242
Garagna S, Marziliano N, Zuccotti M, Searle JB, Capanna E, Redi CA (2001a) Pericentromeric organization at the fusion point of mouse Robertsonian translocation chromosomes. Proc Natl Acad Sci USA 98:171–175
Garagna S, Zuccotti M, Thornhill A, Fernandez-Donoso R, Berrios S, Capanna E, Redi CA (2001b) Alteration of nuclear architecture in male germ cells of chromosomally derived subfertile mice. J Cell Sci 114:4429–4434
Garagna S, Zuccotti M, Capanna E, Redi CA (2002) High-resolution organization of mouse telomeric and pericentromeric DNA. Cytogenet Genome Res 96:125–129
Gazave E, Catalan J, Ramalhinho MG, Mathias ML, Nunes AC, Dumas D, Britton-Davidian J, Auffray J-C (2003) The non-random occurrence of Robertsonian fusion in the house mouse. Genet Res 81:33–42
Geisler M, Gropp A (1967) Chromosome polymorphism in the European hedgehog Erinaceus europaeus (Insectivora). Nature 214:396–397
Giménez MD, White TA, Hauffe HC, Panithanarak T, Searle JB (2013) Understanding the basis of diminished gene flow between hybridizing chromosome races of the house mouse. Evolution 67:1446–1462
Graham AN, Kalitsis P (2014) Chromosome Y centromere array deletion leads to impaired centromere function. PLoS One 9:e86875
Gropp A, Tettenborn U, von Lehmann E (1969) Chromosomenuntersuchungen bei der Tabakmaus (M. poschiavinus) und bei den Hybriden mit der Laboratorumsmaus. Experientia 25:875–876
Gropp A, Winking H, Zech L, Müller H (1972) Robertsonian chromosomal variation and identification of metacentric chromosomes in feral mice. Chromosoma 39:265–288
Guenatri M, Bailly D, Maison C, Almouzni G (2004) Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J Cell Biol 166:493–505
Gündüz İ, López-Fuster MJ, Ventura J, Searle JB (2001) Clinal analysis of a chromosomal hybrid zone in the house mouse. Genet Res 77:41–51
Gündüz İ, Pollock CL, Giménez MD, Förster DW, White TA, Sans-Fuentes MA, Hauffe HC, Ventura J, López-Fuster MJ, Searle JB (2010) Staggered chromosomal hybrid zones in the house mouse: relevance to reticulate evolution and speciation. Genes 1:193–209
Haaf T, Mater AG, Wienberg J, Ward DC (1995) Presence and abundance of CENP-B box sequences in great ape subsets of primate-specific alpha-satellite DNA. J Mol Evol 41:487–491
Handel MA (2004) The XY body: a specialized meiotic chromatin domain. Exp Cell Res 296:57–63
Harper L, Golubovskaya I, Cande WZ (2004) A bouquet of chromosomes. J Cell Sci 117:4025–4032
Hauffe HC, Piálek J (1997) Evolution of the chromosomal races of Mus musculus domesticus in the Rhaetian Alps: the roles of whole-arm reciprocal translocation and zonal raciation. Biol J Linn Soc 62:255–278
Hauffe HC, Searle JB (1992) A disappearing speciation event? Nature 357:26
Hauffe HC, Searle JB (1998) Chromosomal heterozygosity and fertility in house mice (Mus musculus domesticus) from Northern Italy. Genetics 150:1143–1154
Hauffe HC, Giménez MD, Searle JB (2012) Chromosomal hybrid zones in the house mouse. In: Macholán M, Baird SJE, Munclinger P, Piálek J (eds) Evolution of the house mouse. Cambridge University Press, Cambridge, pp 407–430 (Cambridge Series in Morphology and Molecules)
Hochwagen A, Amon A (2006) Checking your breaks: surveillance mechanisms of meiotic recombination. Curr Biol 16:R217–R228
Homer H (2011) New insights into the genetic regulation of homologue disjunction in mammalian oocytes. Cytogenet Genome Res 133:209–222
Homolka D, Ivanek R, Capkova J, Jansa P, Forejt J (2007) Chromosomal rearrangement interferes with meiotic X chromosome inactivation. Genome Res 17:1431–1437
Hori T, Fukagawa T (2012) Establishment of the vertebrate kinetochores. Chromosome Res 20:547–561
Hörz W, Altenburger W (1981) Nucleotide sequence of mouse satellite DNA. Nucleic Acids Res 9:683–696
Hübner R (1992) Chromosomal and biochemical variation in wild mice from Switzerland: relevance for models of chromosomal evolution in European house mice. Unpublished PhD thesis, University of Oxford
Hudson DF, Fowler KJ, Earle E, Saffery R, Kalitsis P, Trowell H, Hill J, Wreford NG, de Kretser DM, Cancilla MR, Howman E, Hii L, Cutts SM, Irvine DV, Choo KH (1998) Centromere protein B null mice are mitotically and meiotically normal but have lower body and testis weights. J Cell Biol 141:309–319
Huet S, Lavelle C, Ranchon H, Carrivain P, Victor J-M, Bancau A (2014) Relevance and limitations of crowding, fractal, and polymer models to describe nuclear architecture. In International Review of Cell and Molecular Biology, Volume 307 (eds H. Ronald and W. J. Kwang), pp. 443–479: Academic Press
Hunt PA, Hassold TJ (2002) Sex matters in meiosis. Science 296:2181–2183
Inagaki A, Schoenmakers S, Baarends WM (2010) DNA double strand break repair, chromosome synapsis and transcriptional silencing in meiosis. Epigenetics 5:255–266
Jachowicz JW, Santenard A, Bender A, Muller J, Torres-Padilla ME (2013) Heterochromatin establishment at pericentromeres depends on nuclear position. Genes Dev 27:2427–2432
Johannisson R, Winking H (1994) Synaptonemal complexes of chains and rings in mice heterozygous for multiple Robertsonian translocations. Chromosome Res 2:137–145
Johannisson R, Winking H (1998) Pachytene chromosomes in trisomy 19 male mice with Robertsonian translocations. Chromosome Res 6:285–294
Joseph A, Mitchell AR, Miller OJ (1989) The organization of the mouse satellite DNA at centromeres. Exp Cell Res 183:494–500
Kalitsis P, Griffiths B, Choo KHA (2006) Mouse telocentric sequences reveal a high rate of homogenization and possible role in Robertsonian translocation. Proc Natl Acad Sci USA 103:8786–8791
Kapoor M, de Oca M, Luna R, Liu G, Lozano G, Cummings C, Mancini M, Ouspenski I, Brinkley BR, May GS (1998) The cenpB gene is not essential in mice. Chromosoma 107:570–576
King M (1993) Species evolution: the role of chromosome change. Cambridge University Press, Cambridge
Kipling D, Cooke HJ (1990) Hypervariable ultra-long telomeres in mice. Nature 347:400–402
Kipling D, Warburton PE (1997) Centromeres, CENP-B and Tigger too. Trends Genet 4:141–145
Kipling D, Ackford HE, Taylor BA, Cooke HJ (1991) Mouse minor satellite DNA genetically maps to the centromere and is physically linked to the proximal telomere. Genomics 11:235–241
Kipling D, Wilson HE, Mitchell AR, Taylor BA, Cooke HJ (1994) Mouse centromere mapping using oligonucleotide probes that detect variants of the minor satellite. Chromosoma 103:46–55
Kipling D, Mitchell AR, Masumoto H, Wilson HE, Nicol L, Cooke HJ (1995) CENP-B binds a novel centromeric sequence in the Asian mouse Mus caroli. Mol Cell Biol 15:4009–4020
Komissarov AS, Gavrilova EV, Demin SJ, Ishov AM, Podgornaya OI (2011) Tandemly repeated DNA families in the mouse genome. BMC Genomics 28(12):531
Kouznetsova A, Wang H, Bellani M, Camerini-Otero RD, Jessberger R, Hoog C (2009) BRCA1-mediated chromatin silencing is limited to oocytes with a small number of asynapsed chromosomes. J Cell Sci 122:2446–2452
Lamond AI, Earnshaw WC (1998) Structure and function in the nucleus. Science 280:547–553
Lanctot C, Cheutin T, Cremer M, Cavalli G, Cremer T (2007) Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat Rev Genet 8:104–115
LeMaire-Adkins R, Radke K, Hunt PA (1997) Lack of checkpoint control at the metaphase/anaphase transition: a mechanism of meiotic nondisjunction in mammalian females. J Cell Biol 139:1611–1619
Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, Jessberger R, Kirkwood TB, Höög C, Herbert M (2010) Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 20:1511–1521
Long JA (1908) Some maturation stages of the mouse egg. Science 27:443–444
Longo F, Garagna S, Merico V, Orlandini G, Gatti R, Scandroglio R, Redi CA, Zuccotti M (2003) Nuclear localization of NORs and centromeres in mouse oocytes during folliculogenesis. Mol Reprod Dev 66:279–290
Mahadevaiah SK, Bourc’his D, de Rooij DG, Bestor TH, Turner JM, Burgoyne PS (2008) Extensive meiotic asynapsis in mice antagonises meiotic silencing of unsynapsed chromatin and consequently disrupts meiotic sex chromosome inactivation. J Cell Biol 182:263–276
Manterola M, Page J, Vasco C, Berrios S, Parra MT, Viera A, Rufas JS, Zuccotti M, Garagna S, Fernandez-Donoso R (2009) A high incidence of meiotic silencing of unsynapsed chromatin is not associated with substantial pachytene loss in heterozygous male mice carrying multiple simple Robertsonian translocations. PLoS Genet 5:e1000625
Medarde N, López-Fuster MJ, Mũnoz-Mũnoz F, Ventura J (2012) Spatio-temporal variation in the structure of a chromosomal polymorphism zone in the house mouse. Heredity 109:78–89
Merico V, Pigozzi MI, Esposito A, Merani MS, Garagna S (2003) Meiotic recombination and spermatogenic impairment in Mus musculus domesticus carrying multiple simple Robertsonian translocations. Cytogenet Genome Res 103:321–329
Merico V, de Barboza GD, Vasco C, Ponce R, Rodriguez V, Garagna S, de Talamoni NT (2008) A mitochondrial mechanism is involved in apoptosis of Robertsonian mouse male germ cells. Reproduction 135:797–804
Merico V, Giménez MD, Vasco C, Zuccotti M, Searle JB, Hauffe HC, Garagna S (2013) Chromosomal speciation in mice: a cytogenetic analysis of recombination. Chromosome Res 21:523–533
Mitsainas GP, Giagia-Athanasopoulou EB (2005) Studies on the Robertsonian chromosomal variation of Mus musculus domesticus (Rodentia, Muridae) in Greece. Biol J Linn Soc 84:503–513
Mitsainas GP, Giagia-Athanasopoulou EB (2009) Possible involvement of whole-arm reciprocal translocations (WARTs) in the evolution of a Mus musculus domesticus Robertsonian system from Greece. Rend Fis Acc Lincei 20:153–162
Mlynarski EE, Obergfell CJ, O’Neill MJ, O’Neill RJ (2010) Divergent patterns of breakpoint reuse in Muroid rodents. Mamm Genome 21:77–87
Muro Y, Masumoto H, Yoda K, Nozaki N, Ohashi M, Okazaki T (1992) Centromere protein B assembles human centromeric alpha-satellite DNA at the 17-bp sequence, CENP-B box. J Cell Biol 116:585–596
Nachman MW, Searle JB (1995) Why is the house mouse karyotype so variable? Trends Ecol Evol 10:397–402
Nancé V, Vanlerberghe F, Nielsen JT, Bonhomme F, Britton-Davidian J (1990) Chromosomal introgression in house mice from the hybrid zone between M. m. domesticus and M. m. musculus in Denmark. Biol J Linn Soc 41:215–227
Nanda I, Schneider-Rasp S, Winking H, Schmid M (1995) Loss of telomeric sites in the chromosomes of Mus musculus domesticus (Rodentia: Muridae) during Robertsonian rearrangements. Chromosome Res 3:399–409
Naumova AK, Fayer S, Leung J, Boateng KA, Camerini-Otero RD, Taketo T (2013) Dynamics of response to asynapsis and meiotic silencing in spermatocytes from Robertsonian translocation carriers. PLoS One 8:e75970
Navarro J, Vidal F, Benet J, Templado C, Marina S, Egozcue J (1991) XY-trivalent association and synaptic anomalies in a male carrier of a Robertsonian t(13;14) translocation. Hum Reprod 6:376–381
Neale MJ, Keeney S (2006) Clarifying the mechanics of DNA strand exchange in meiotic recombination. Nature 442:153–158
Nguyen TT, Aniskin VM, Gerbault-Seureau M, Planton H, Renard JP, Nguyen BX, Hassanin A, Volobouev VT (2008) Phylogenetic position of the saola (Pseudoryx nghetinhensis) inferred from cytogenetic analysis of eleven species of Bovidae. Cytogenet Genome Res 122:41–54
Ohzeki J, Nakano M, Okada T, Masumoto H (2002) CENP-B box is required for de novo centromere chromatin assembly on human alphoid DNA. J Cell Biol 159:765–775
Okada T, Ohzeki J, Nakano M, Yoda K, Brinkley WR, Larionov V, Masumoto H (2007) CENP-B controls centromere formation depending on the chromatin context. Cell 131:1287–1300
Page J, de la Fuente R, Manterola M, Parra MT, Viera A, Berrios S, Fernandez-Donoso R, Rufas JS (2012) Inactivation or non-reactivation: what accounts better for the silence of sex chromosomes during mammalian male meiosis? Chromosoma 121:307–326
Painter TS (1928) A comparison of the chromosomes of the rat and mouse with reference to the question of chromosome homology in mammals. Genetics 13:180–189
Panithanarak T, Hauffe HC, Dallas JF, Glover A, Ward RG, Searle JB (2004) Linkage-dependent gene flow in a house mouse chromosomal hybrid zone. Evolution 58:184–192
Pardo-Manuel de Villena F, Sapienza C (2001) Female meiosis drives karyotypic evolution in mammals. Genetics 159:1179–1189
Pardue ML, Gall JG (1970) Chromosomal localization of mouse satellite DNA. Science 168:1356–1358
Perez-Castro AV, Shamanski FL, Meneses JJ, Lovato TL, Vogel KG, Moyzis RK, Pedersen R (1998) Centromeric protein B null mice are viable with no apparent abnormalities. Dev Biol 201:135–143
Pertile MD, Graham AN, Choo KHA, Kalitsis P (2009) Rapid evolution of mouse Y centromere repeat DNA belies recent sequence stability. Genome Res 19:2202–2213
Piálek J, Hauffe HC, Searle JB (2005) Chromosomal variation in the house mouse. Biol J Linn Soc 84:535–563
Pietras DF, Bennett KL, Siracusa LD, Woodworth-Gutai M, Chapman VM, Gross KW, Kane-Haas C, Hastie ND (1983) Construction of a small Mus musculus repetitive DNA library: identification of a new satellite sequence in Mus musculus. Nucleic Acids Res 11:6965–6983
Plohl M, Luchetti A, Mestrović N, Mantovani B (2008) Satellite DNAs between selfishness and functionality: structure, genomics and evolution of tandem repeats in centromeric (hetero)chromatin. Gene 409:72–82
Rebuzzini P, Castiglia R, Nergadze SG, Mitsainas G, Munclinger P, Zuccotti M, Capanna E, Redi CA, Garagna S (2009) Quantitative variation of LINE-1 sequences in five species and three subspecies of the subgenus Mus and in five Robertsonian races of Mus musculus domesticus. Chromosome Res 17:65–76
Redi CA, Capanna E (1988) Robertsonian heterozygotes in the house mouse and the fate of their germ cells. In: Daniel A (ed) The cytogenetics of mammalian autosomal rearrangements. Liss, New York, pp 315–359
Redi CA, Garagna S, Hilscher B, Winking H (1985) The effects of some Robertsonian chromosome combinations on the seminiferous epithelium of the mouse. J Embryol Exp Morphol 85:1–19
Redi CA, Garagna S, Della Valle G, Bottiroli G, Dell’Orto P, Viale G, Peverali FA, Raimondi E, Forejt J (1990) Differences in the organization and chromosomal allocation of satellite DNA between the European long tailed house mice Mus domesticus and Mus musculus. Chromosoma 99:11–17
Rieseberg LH (2001) Chromosomal rearrangements and speciation. Trends Ecol Evol 16:351–358
Roeder GS, Bailis JM (2000) The pachytene checkpoint. Trends Genet 16:395–403
Royo H, Polikiewicz G, Mahadevaiah SK, Prosser H, Mitchell M, Bradley A, de Rooij DG, Burgoyne PS, Turner JM (2010) Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr Biol 20:2117–2123
Sage RD, Atchley WR, Capanna E (1993) House mice as models in systematic biology. Syst Biol 42:523–561
Saïd K, Jacquart T, Montgelard C, Sonjaya H, Helal AN, Britton-Davidian J (1986) Robertsonian house mouse populations in Tunisia: a karyological and biochemical study. Genetica 68:151–156
Sasaki N, Yamauchi H, Tomohiro N, Agui T (2013) The telocentric tandem repeat at the p-arm is not conserved in Mus musculus subspecies. Gene 513:214–218
Scherthan H (2007) Telomeres and meiosis in health and disease. Cell Mol Life Sci 64:117–124
Schimenti J (2005) Synapsis or silence. Nat Genet 37:11–13
Searle JB (1990) A cytogenetic analysis of reproduction in common shrews (Sorex araneus) from a karyotypic hybrid zone. Hereditas 113:121–132
Searle JB (1993) Chromosomal hybrid zones in eutherian mammals. In: Harrison RG (ed) Hybrid zones and the evolutionary process. Oxford University Press, New York, pp 309–353
Searle JB, Hübner R, Wallace BMN, Garagna S (1990) Robertsonian variation in wild mice and shrews. Chromosomes Today 10:253–263
Solano E, Castiglia R, Corti M (2007) A new chromosomal race of the house mouse, Mus musculus domesticus, in the Vulcano Island-Aeolian Archipelago, Italy. Hereditas 144:75–77
Solano E, Castiglia R, Capanna E (2009) Chromosomal evolution of the house mouse, Mus musculus domesticus, in the Aeolian Archipelago (Sicily, Italy). Biol J Linn Soc 96:194–202
Szamalek JM, Goidts V, Searle JB, Cooper DN, Hameister H, Kehrer-Sawatzki H (2006) The chimpanzee-specific pericentric inversions that distinguish humans and chimpanzees have identical breakpoints in Pan troglodytes and Pan paniscus. Genomics 87:39–45
Tanaka Y, Tachiwana H, Yoda K, Masumoto H, Okazaki T, Kurumizaka H, Yokoyama S (2005) Human centromere protein B induces translational positioning of nucleosomes on alpha-satellite sequences. J Biol Chem 280:41609–41618
Therman E, Susman M (1993) Human chromosomes: structure, behavior, and effects, 3rd edn. Springer Verlag, New York
Tichy H, Vucak I (1987) Chromosomal polymorphism in the house mouse (Mus domesticus) of Greece and Yugoslavia. Chromosoma 95:31–36
Tomascik-Cheeseman L, Marchetti F, Lowe X, Shamanski FL, Nath J, Pedersen RA, Wyrobek AJ (2002) CENP-B is not critical for meiotic chromosome segregation in male mice. Mutat Res 513:197–203
Trifonov VA, Kosyakova N, Romanenko SA, Stanyon R, Graphodatsky AS, Liehr T (2010) New insights into the karyotypic evolution in muroid rodents revealed by multicolor banding applying murine probes. Chromosome Res 18:265–275
Turner JM (2007) Meiotic sex chromosome inactivation. Development 134:1823–1831
Turner JM, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GV, Barrett JC, Burgoyne PS, Deng CX (2004) BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol 14:2135–2142
Turner JM, Mahadevaiah SK, Fernandez-Capetillo O, Nussenzweig A, Xu X, Deng CX, Burgoyne PS (2005) Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 37:41–47
Turner JM, Mahadevaiah SK, Ellis PJ, Mitchell MJ, Burgoyne PS (2006) Pachytene asynapsis drives meiotic sex chromosome inactivation and leads to substantial postmeiotic repression in spermatids. Dev Cell 10:521–529
Vasco C, Manterola M, Page J, Zuccotti M, de la Fuente R, Redi CA, Fernandez-Donoso R, Garagna S (2012) The frequency of heterologous synapsis increases with aging in Robertsonian heterozygous male mice. Chromosome Res 20:269–278
Veyrunes F, Dobigny G, Yang F, O’Brien PC, Catalan J, Robinson TJ, Britton-Davidian J (2006) Phylogenomics of the genus Mus (Rodentia; Muridae): extensive genome repatterning is not restricted to the house mouse. Proc R Soc B 273:2925–2934
Vissel B, Choo KHA (1989) Mouse major (gamma) satellite DNA is highly conserved and organized into extremely long tandem arrays: implications for recombination between nonhomologous chromosomes. Genomics 5:407–414
Vogt E, Kirsch-Volders M, Parry J, Eichenlaub-Ritter U (2008) Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat Res 651:14–29
Volobouev V, Vogt N, Viegas-Péquignot E, Malfoy B, Dutrillaux B (1995) Characterization and chromosomal location of two repeated DNAs in three Gerbillus species. Chromosoma 104:252–259
Wallace BMN, Searle JB (1994) Oogenesis in homozygotes and heterozygotes for Robertsonian rearrangements from natural populations of the common shrew, Sorex araneus. J Reprod Fertil 100:231–237
Wallace BMN, Searle JB, Everett CA (1992) Male meiosis and gametogenesis in wild house mice (Mus musculus domesticus) from a chromosomal hybrid zone; a comparison between “simple” Robertsonian heterozygotes and homozygotes. Cytogenet Cell Genet 61:211–220
Wallace BMN, Searle JB, Everett CA (2002) The effect of multiple simple Robertsonian heterozygosity on chromosome pairing and fertility of wild-stock house mice (Mus musculus domesticus). Cytogenet Genome Res 96:276–286
White TA, Bordewich M, Searle JB (2010) A network approach to study karyotypic evolution: the chromosomal races of the common shrew (Sorex araneus) and house mouse (Mus musculus) as a model systems. Syst Biol 59:262–276
Winking H, Reuter C, Bostelmann H (2000) Unequal nondisjunction frequencies of trivalent chromosomes in male mice heterozygous for two Robertsonian translocations. Cytogenet Genome Res 91:303–306
Wójcik JM, Searle JB (1988) The chromosome complement of Sorex granarius—the ancestral karyotype of the common shrew (Sorex araneus)? Heredity 61:225–229
Wong AK, Rattner JB (1988) Sequence organization and cytological localization of the minor satellite of mouse. Nucleic Acids Res 16:11645–11661
Yang F, Carter NP, Shi L, Ferguson-Smith MA (1995) A comparative study of karyotypes of muntjacs by chromosome painting. Chromosoma 103:642–652
Yang H, Wang JR, Didion JP, Buus RJ, Bell TA, Welsh CE, Bonhomme F, Yu AH, Nachman MW, Piálek J, Tucker P, Boursot P, McMillan L, Churchill GA, de Villena FP (2011) Subspecific origin and haplotype diversity in the laboratory mouse. Nat Genet 43:648–655
Yasmineh WG, Yunis JJ (1969) Satellite DNA in mouse autosomal heterochromatin. Biochem Biophys Res Comm 35:779–782
Zeng K, de las Heras JI, Ross A, Yang J, Cooke H, Shen MH (2004) Localisation of centromeric proteins to a fraction of mouse minor satellite DNA on a mini-chromosome in human, mouse and chicken cells. Chromosoma 113:84–91
Zima J, Gaichenko VA, Macholan M, Radjabli SI, Sablina OV, Wójcik JM (1990) Are Robertsonian variations a frequent phenomenon in mouse populations in Eurasia? Biol J Linn Soc 41:229–233
Acknowledgments
We thank the many colleagues and students who have worked with us on the Robertsonian phenomenon. We are indebted to Ernesto Capanna for his generous gift of the picture of him and Alfred Gropp. We are grateful to the institutions and foundations that have contributed funding for the development of our work. S.G. was supported by Fondo di Ateneo per la Ricerca, University of Pavia (Italy); M.Z. was supported by Finanziamento Ricerca Locale, University of Parma (Italy); J.P. was supported by grant BFU2009/10987 from Ministerio de Ciencia e Innovación (Spain); and R.F.D. was supported by FONDECYT grant 1120160 (Chile).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garagna, S., Page, J., Fernandez-Donoso, R. et al. The Robertsonian phenomenon in the house mouse: mutation, meiosis and speciation. Chromosoma 123, 529–544 (2014). https://doi.org/10.1007/s00412-014-0477-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-014-0477-6