Abstract
Background
Vascular endothelial growth factor (VEGF) is an important stimulator of choroidal neovascularization (CNV). Bevacizumab (Avastin), ranibizumab (Lucentis) and pegaptanib sodium (Macugen) are anti-VEGF medications that have been used in the treatment of CNV. The purpose of our study is to evaluate the efficacy and safety of intravitreal injections of bevacizumab, ranibizumab and pegaptanib sodium in the treatment of CNV in a rat model.
Methods
Multiple CNV lesions were induced by laser photocoagulation of the retina in Brown-Norway rats. After 3 weeks, 17 rats were divided into three groups and received intravitreal injections of bevacizumab, ranibizumab or pegaptanib sodium in different dosages. The lesions were evaluated by fluorescein angiography 1, 7, 14, and 28 days later to assess the efficacy of these medications.
Results
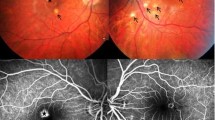
Different doses of bevacizumab did not show any effect on stopping the leakage on fluorescein angiography on days 1, 7, 14, and 28. Ranibizumab and pegaptanib sodium did not stop the leakage of CNV either. No angiographic or histopathologic toxicity was observed.
Conclusions
These three anti-VEGF agents did not show any therapeutic effect on stopping CNV leakage in rats. Previous experiments with ranibizumab in monkeys resulted in a significant decrease in leakage of CNV. The difference may be due to the fact that both ranibizumab and bevacizumab are humanized and species-specific. There are several studies evaluating the effect of bevacizumab in non-primates. Since bevacizumab is humanized, the results of studies on non-primates may not be similar to humans and non-human primates.






Similar content being viewed by others
References
Kliffen M, Sharma HS, Mooy HS, Kerkvliet CM, de Jong PTVM (1997) Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 81:154–162
Bashshur ZF, Haddad ZA, Schakal A, Jaafar RF, Saab M, Noureddin, BN (2008) Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: a one-year prospective study. Am J Ophthalmol 145(2):249–256
Klein R, Klein BE, Jensen SC, Meuer SM (1997) The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 104:7–21
Weiss M, Roth DB, Prenner JL (2007) The incidence of systemic adverse events in patients treated with intravitreal bevacizumab (Avastin). Invest Ophthalmol Vis Sci 48:4939
Ameri H, Chader GJ, Kim J, Sadda SR, Rao NA, Humayun MS (2007) The effects of intravitreous bevacizumab on retinal neovascular membrane and normal capillaries in rabbits. Invest Ophthalmol Vis Sci 48:5708–5715
Manzano RP, Peyman GA, Khan P, Carvounis PE, Kivilcim M, Ren M, Lake JC, Chévez-Barrios P (2007) Inhibition of experimental corneal neovascularization by bevacizumab (Avastin). Br J Ophthalmol 91:804–807
Barros LM, Belford R Jr (2007) The effect of the subconjunctival injection of bevacizumab (Avastin) on angiogenesis in the rat cornea. An Acad Bras Cienc 79:389–394
Bock F, Onderka J, Dietrich T, Bachmann B, Kruse FE, Paschke M, Zahn G, Cursiefen C (2007) Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci 48:2545–2552
Heiduschka P, Fietz H, Hofmeister S, Schultheiss S, Mack AF, Peters S, Ziemssen F, Niggemann B, Julien S, Bartz-Schmidt KU, Schraermeyer U, The Tübingen Bevacizumab Study Group (2007) Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Invest Ophthalmol Vis Sci 48:2814–2823
Krzystolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES, Michaud NA, Li W, Connolly E, O’Neill CA, Miller JW (2002) Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol 120:338–346
Yu L, Wu X, Cheng Z, Lee CV, LeCouter J, Campa C, Fuh G, Lowman H, Ferrara N (2008) Interaction between bevacizumab and murine VEGF-A: A reassessment. Invest Ophthalmol Vis Sci 49:522–527
Zacks DN, Ezra E, Terada Y, Michaud N, Connolly E, Gragoudas ES, Miller JW (2002) Verteporfin photodynamic therapy in the rat model of choroidal neovascularization: angiographic and histologic characterization. Invest Ophthalmol Vis Sci 43:2384–2391
Ciulla TA, Criswell MH, Danis RP, Hill TE (2001) Intravitreal triamcinolone acetonide inhibits choroidal neovascularization in a laser-treated rat model. Arch Ophthalmol 119:399–404
Terada Y, Michaud NA, Connolly EJ, Lane A, Ohtsuki H, Gragoudas ES, Miller JW (2003) Enhanced photodynamic therapy using angiostatin with verteporfin PDT in a laser-injury rat model. Invest Ophthalmol Vis Sci 44:1749
Takehana Y, Kurokawa T, Kitamura T, Tsukahara Y, Akahane S, Kitazawa M, Yoshimura N (1999) Suppression of laser-induced choroidal neovascularization by oral tranilast in the rat. Invest Ophthalmol Vis Sci 40:459–466
Berkowitz BA, Lukaszew RA, Mullins CM, Penn JS (1998) Impaired hyaloidal circulation function and uncoordinated ocular growth patterns in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci 39:391–397
Hughes A (1979) A schematic eye for the rat. Vision Res 19:569–588
Bikfalvi A, Sauzeau C, Moukadiri H, Maclouf J, Busso N, Bryckaert M, Plouet J, Tobelem G (1991) Interaction of vasculotropin/vascular endothelial cell growth factor with human umbilical vein endothelial cells: binding, internalization, degradation, and biological effects. J Cell Physiol 149:50–59
Vaisman N, Gospodarowicz D, Neufeld G (1990) Characterization of the receptors for vascular endothelial growth factor. J Biol Chem 265:19461–19466
Plouet J, Moukadiri H (1990) Characterization of the receptor to vasculotropin on bovine adrenal cortex-derived capillary endothelial cells. J Biol Chem 265:22071–22074
Kwak N, Okamoto N, Wood JM, Campochiaro PA (2000) VEGF is major stimulator in model of choroidal neovascularization. Invest Ophthalmol Vis Sci 41:3158–3164
Kim KJ, Li B, Houck K, Winer J, Ferrara N (1992) The vascular endothelial growth factor proteins: identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors 7:53–64
Fuh G, Wu P, Liang WC, Ultsch M, Lee CV, Moffat B, Wiesmann C (2006) Structure-function studies of two synthetic anti-vascular endothelial growth factor Fabs and comparison with the Avastin Fab. J Biol Chem 281:6625–6631
Campochiaro PA, The First ARVO/Pfizer Institute Working Group (2006) Ocular versus extraocular neovascularization: mirror images or vague resemblances. Invest Ophthalmol Vis Sci 47:462–474
Acknowledgement
The authors would like to acknowledge John Sinard, MD, PhD, for his advice regarding pathology slides.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant Support: Leir Foundation
The authors have full control of the primary data and will provide it to Graefe’s Archive for Clinical and Experimental Ophthalmology at their request. Neither author has any conflict of interest, financial or otherwise, to report.
Rights and permissions
About this article
Cite this article
Lu, F., Adelman, R.A. Are intravitreal bevacizumab and ranibizumab effective in a rat model of choroidal neovascularization?. Graefes Arch Clin Exp Ophthalmol 247, 171–177 (2009). https://doi.org/10.1007/s00417-008-0936-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-0936-y