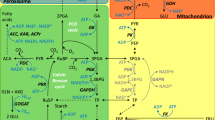
Abstract
Mitochondria isolated from the roots of barley (Hordeum vulgare L.) and rice (Oryza sativa L.) seedlings were capable of oxidizing external NADH and NADPH anaerobically in the presence of nitrite. The reaction was linked to ATP synthesis and nitric oxide (NO) was a measurable product. The rates of NADH and NADPH oxidation were in the range of 12–16 nmol min−1 mg−1 protein for both species. The anaerobic ATP synthesis rate was 7–9 nmol min−1 mg−1 protein for barley and 15–17 nmol min−1 mg−1 protein for rice. The rates are of the same order of magnitude as glycolytic ATP production during anoxia and about 3–5% of the aerobic mitochondrial ATP synthesis rate. NADH/NADPH oxidation and ATP synthesis were sensitive to the mitochondrial inhibitors myxothiazol, oligomycin, diphenyleneiodonium and insensitive to rotenone and antimycin A. The uncoupler FCCP completely eliminated ATP production. Succinate was also capable of driving ATP synthesis. We conclude that plant mitochondria, under anaerobic conditions, have a capacity to use nitrite as an electron acceptor to oxidize cytosolic NADH/NADPH and generate ATP.






Similar content being viewed by others
Abbreviations
- AOX:
-
Alternative oxidase
- COX:
-
Cytochrome c oxidase
- DPI:
-
Diphenyleneiodonium
- ETC:
-
Electron transport chain
- FCCP:
-
Carbonylcyanide-p-trifluoromethoxyphenylhydrazone
- Hb:
-
Hemoglobin
References
Affourtit C, Krab K, Moore AL (2001) Control of plant mitochondrial respiration. Biochim Biophys Acta 1504:58–69
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brewer GJ, Jones TT, Wallimann T, Schlattner U (2004) Higher respiratory rates and improved creatine stimulation in brain mitochondria isolated with anti-oxidants. Mitochondrion 4:49–57
Brown GC, Cooper CE (1994) Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 356:295–298
Brudvig GW, Stevens TH, Chan SI (1980) Reactions of nitric oxide with cytochrome c oxidase. Biochemistry 19:5275–5285
Castello PR, David PS, McClure T, Crook Z, Poyton RO (2006) Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metabolism 3:277–287
Cooper CE (2002) Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector? Trends Biochem Sci 27:33–39
Cooper CE (2003) Competitive, reversible, physiological? Inhibition of mitochondrial cytochrome oxidase by nitric oxide. IUBMB Life 55:591–597
Couee I, Defontaine S, Carde JP, Pradet A (1992) Effects of anoxia on mitochondrial biogenesis in rice shoots – modification of in organello translation characteristics. Plant Physiol 98:411–421
Day D, Wiskich JT (1977) Factors limiting respiration by isolated cauliflower mitochondria. Phytochemistry 16:1499–1502
Dordas C, Hasinoff BB, Igamberdiev AU, Manac’h N, Rivoal J, Hill RD (2003) Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J 35:763–770
Douce R (1985) Mitochondria in higher plants. structure, function, and biogenesis. Academic, Orlando
Escobar MA, Geisler DA, Rasmusson AG (2006) Reorganization of the alternative pathways of the Arabidopsis respiratory chain by nitrogen supply: opposing effects of ammonium and nitrate. Plant J 45:775–788
Finlay BJ, Span ASW, Harman JMP (1983) Nitrate respiration in primitive eukaryotes. Nature 303:333–336
Fox TC, Kennedy RA (1991) Mitochondrial enzymes in aerobically and anaerobically germinated seedlings of Echinochloa and rice. Planta 184:510–514
Geigenberger P, Fernie AR, Gibon Y, Christ M, Stitt M (2000) Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biol Chem 381:723–740
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30:1–47
Greenway H, Gibbs J (2004) Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Funct Plant Biol 30:999–1036
Guo FQ, Crawford NM (2005) Arabidopsis nitric oxide synthase 1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 17:3436–3450
Gupta KJ, Stoimenova M, Kaiser WM (2005) In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot 56:2601–2609
Hageman RH, Reed AJ (1980) Nitrate reductase from higher plants. Methods Enzymol 69:270–280
Howell KA, Millar AH, Whelan J (2006) Ordered assembly of mitochondria during rice germination begins with promitochondrial structures rich in components of the protein import apparatus. Plant Molec Biol 60:201–223
Huang X, von Rad U, Durner J (2002) Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta 215:914–923
Igamberdiev AU, Baron K, Manac’h N, Stoimenova M, Hill RD (2005) The haemoglobin/nitric oxide cycle: involvement in flooding stress and effects on hormone signalling. Ann Bot 96:557–564
Igamberdiev AU, Bykova NV, Hill RD (2006) Scavenging of nitric oxide by barley hemoglobin is facilitated by a monodehydroascorbate reductase-mediated ascorbate reduction of methemoglobin. Planta 223:1033–1040
Igamberdiev AU, Hill RD (2004) Nitrate, NO and haemoglobin in plant adaptation to hypoxia: An alternative to classic fermentation pathways. J Exp Bot 55:2473–2482
Igamberdiev AU, Seregélyes C, Manac’h N, Hill RD (2004) NADH-dependent metabolism of nitric oxide in alfalfa root cultures expressing barley hemoglobin. Planta 219:95–102
Kennedy RA, Fox TC, Siedow JN (1987) Activities of isolated mitochondria and mitochondrial enzymes from aerobically and anaerobically germinated barnyard grass (Echinochloa) seedlings. Plant Physiol 85:474–480
Kennedy RA, Rumpho ME, Fox TC (1992) Anaerobic metabolism in plants. Plant Physiol 100:1–6
Kobayashi M, Matsuo Y, Takimoto A, Suzuki S, Maruo F, Shoun H (1996) Denitrification, a novel type of respiratory metabolism in fungal mitochondrion. J Biol Chem 271:16263–16267
Kozlov AV, Staniek K, Nohl H (1999) Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett 454:127–130
Kozlov AV, Costantino G, Sobhian B, Szalay L, Umar F, Nohl H, Bahrami S, Redl H (2005) Mechanisms of vasodilatation induced by nitrite instillation in intestinal lumen: possible role of hemoglobin. Antioxid Redox Signal 7:515–521
Lasca Z, Kozlov AV, Pankotai E, Csordás A, Wolf G, Redl H, Kollai M, Szabó C, Busija DW, Horn TFW (2006) Mitochondria produce reactive nitrogen species via an arginine-independent pathway. Free Radical Res 40:369–378
Logan DC, Millar AH, Sweetlove LJ, Hill SA, Leaver CJ (2001) Mitochondrial biogenesis during germination in maize embryos. Plant Physiol 125:662–672
Mason MG, Nicholls P, Wilson MT, Cooper CE (2006) Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc Natl Acad Sci USA 103:708–713
Millar AH, Bergensen FJ, Day DA (1994) Oxygen affinity of terminal oxidases in soybean mitochondria. Plant Physiol Biochem 32:847–852
Møller IM (1997) The oxidation of cytosolic NAD(P)H by external NAD(P)H dehydrogenases in the respiratory chain of plant mitochondria. Physiol Plant 100:85–90
Møller IM, Lin W (1986) Membrane-bound NAD(P)H dehydrogenases in higher plant cells. Annu Rev Plant Physiol 37:309–334
Muller F, Crofts AR, Kramer DM (2002) Multiple Q-cycle bypass reactions at the Qo site of the cytochrome bc 1 complex. Biochemistry 41:7866–7874
Neuburger M, Rébéille F, Jourdain A, Nakamura S, Douce R (1996) Mitochondria are a major site for folate and thymidilate synthesis in plants. J Biol Chem 271:9466–9472
Nichols JW, Weber LJ (1989) Comparative oxygen affinity of fish and mammalian hemoglobins. J Comp Physiol B 159:205–209
Nishimura M, Douce R, Akazawa T (1982) Isolation and characterization of metabolically competent mitochondria from spinach leaf protoplasts. Plant Physiol 69:916–920
Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, Cantoni O, Carruba MO, Moncada S, Clementi E (2004) Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA 101:16507–16512
Nohl H, Kozlov AV, Staniek K, Gille L (2001) The multiple functions of coenzyme Q. Bioorg Chem 29:1–13
Paitian NA, Markossian KA, Nalbandyan RM (1985) The effect of nitrite on cytochrome oxidase. Biochem Biophys Res Commun 133:1104–1111
Pearce LL, Kanai AJ, Birder LA, Pitt BR, Peterson J (2002) The catabolic fate of nitric oxide: the nitric oxide oxidase and peroxynitrite reductase activities of cytochrome oxidase. J Biol Chem 277:13556–13562
Planchet E, Gupta KJ, Sonoda M, Kaiser WM (2005) Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J 41:732–743
Polyakova LI, Vartapetian BB (2003) Exogenous nitrate as a terminal acceptor of electrons in rice (Oryza sativa) coleoptiles and wheat (Triticum aestivum) roots under strict anoxia. Russian J Plant Physiol 50:808–812
Richter C (1997) Reactive oxygen and nitrogen species regulate mitochondrial Ca2+ homeostasis and respiration. Biosci Rep 17:53–66
Roberts TH, Fredlund KM, Møller IM (1995) Direct evidence for the presence of two external NAD(P)H dehydrogenases coupled to the electron transport chain in plant mitochondria. FEBS Lett 373:307–309
Siedow JN, Umbach AL (1995) Plant mitochondrial electron transfer and molecular biology. Plant Cell 7:821–831
Sowa AW, Duff SMG, Guy PA, Hill RD (1998) Altering hemoglobin levels changes energy status in maize cells under hypoxia. Proc Natl Acad Sci USA 95:10317–10321
Stouthamer AH (1991) Metabolic regulation including anaerobic metabolism in Paracoccus denitrificans. J Bioenerg Biomembr 23:163–185
Subbaiah CC, Bush DS, Sachs MM (1998) Mitochondrial contribution to the anoxic Ca2+ signal in maize suspension-cultured cells. Plant Physiol 118:759–771
Tielens AGM, Rotte C, van Hellemond JJ, Martin W (2002) Mitochondria as we don’t know them. Trends Biochem Sci 27:564–572
Tischner R, Planchet E, Kaiser WM (2004) Mitochondrial electron transport as a source for nitric oxide in the unicellular green alga Chlorella sorokiniana. FEBS Lett 576:151–155
Vanlerberghe GC, Day DA, Wiskich JT, Vanlerberghe AE, McIntosh L (1995) Alternative oxidase activity in tobacco leaf mitochondria (dependence on tricarboxylic acid cycle-mediated redox regulation and pyruvate activation). Plant Physiol 109:353–361
Vartapetian BB, Polyakova LI (1999) Protective effect of exogenous nitrate on the mitochondrial ultrastructure of Oryza sativa coleoptiles under strict anoxia. Protoplasma 206:163–167
Vartapetian BB, Andreeva IN, Generozova IP, Polyakova LI, Maslova IP, Dolgikh YI, Stepanova AY (2003) Functional electron microscopy in studies of plant response and adaptation to anaerobic stress. Ann Bot 91:155–172
Walters CL, Taylor AM (1965) The reduction of nitrite by skeletal muscle mitochondria. Biochim Biophys Acta 522–524
Yamasaki H, Sakihama Y (2000) Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett 468:89–92
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Molec Biol Rev 61:533–616
Acknowledgments
We thank Werner M. Kaiser for his cooperation and helpful suggestions. This work was supported by the Natural Sciences and Engineering Research Council of Canada and Genome Canada (to R.D.H.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stoimenova, M., Igamberdiev, A.U., Gupta, K.J. et al. Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta 226, 465–474 (2007). https://doi.org/10.1007/s00425-007-0496-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0496-0