Abstract
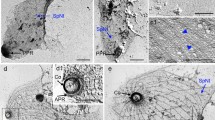
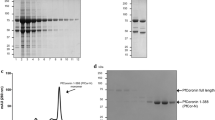
After host cell invasion, Toxoplasma secretes a variety of dense granule proteins (GRA proteins) from its secretory dense granules, which are involved in the biogenesis of the parasitophorous vacuole (PV). TgGRA8I is predicted to contain proline-rich domains, which are structural features of some cytoskeleton-related proteins. In agreement with this observation, previous proteomic analyses revealed the presence of TgGRA8I in the Toxoplasma sub-pellicular cytoskeleton. In the present study, we show (1) by docking analyses that TgGRA8I may interact with both Toxoplasma β-tubulin and actin; (2) by immunoelectron microscopy, proteomic, biochemical, and cellular approaches that TgGRA8I associates with sub-pellicular microtubules and actin at the parasite sub-pellicular cytoskeleton; (3) that type I parasites (RH strain) lacking the GRA8 gene (RHΔku80Δgra8) exhibit loss of conoid extrusion, diminished cell infection, and egress capabilities, and that these motility impairments were likely due to important alterations in their sub-pellicular cytoskeleton, in particular their sub-pellicular microtubules and meshwork. Parasites lacking the GRA4 gene (RHΔku80Δgra4) did not show modifications in the organization of the sub-pellicular cytoskeleton. Collectively, these results demonstrated that TgGRA8I is a dense granule protein that, besides its role in the formation of the PV, contributes to the organization of the parasite sub-pellicular cytoskeleton and motility. This is the first proline-rich protein described in the Toxoplasma cytoskeleton, which is a key organelle for both the parasite motility and the invasion process. Knowledge about the function of cytoskeleton components in Toxoplasma is fundamental to understand the motility process and the host cell invasion mechanism. Refining this knowledge should lead to the design of novel pharmacological strategies for the treatment against toxoplasmosis.







Similar content being viewed by others
References
Anderson-White BR, Ivey FD, Cheng K, Szatanek T, Lorestani A, Beckers CJ, Ferguson DJ, Sahoo N, Gubbels MJ (2011) A family of intermediate filament-like proteins is sequentially assembled into the cytoskeleton of Toxoplasma gondii. Cell Microbiol 13:18–31
Bellanger JM, Cueva JG, Baran R, Tang G, Goodman MB, Debant A (2012) The doublecortin-related gene zyg-8 is a microtubule organizer in Caenorhabditis elegans neurons. J Cell Sci 125:5417–5427
Bougdour A, Tardieux I, Hakimi MA (2014) Toxoplasma exports dense granule proteins beyond the vacuole to the host cell nucleus and rewires the host genome expression. Cell Microbiol 16:334–343
Braun L, Travier L, Kieffer S, Musset K, Garin J, Mercier C, Cesbron-Delauw MF (2008) Purification of Toxoplasma dense granule proteins reveals that they are in complexes throughout the secretory pathway. Mol Biochem Parasitol 157:13–21
Bullen HE, Tonkin CJ, O'Donnell RA, Tham WH, Papenfuss AT, Gould S, Cowman AF, Crabb BS, Gilson PR (2009) A novel family of Apicomplexan glideosome-associated proteins with an inner membrane-anchoring role. J Biol Chem 284:25353–25363
Caldas LA, Seabra SH, Attias M, de Souza W (2013) The effect of kinase, actin, myosin and dynamin inhibitors on host cell egress by Toxoplasma gondii. Parasitol Int 62:475–482
Carey KL, Donahue CG, Ward GE (2000) Identification and molecular characterization of GRA8, a novel, proline-rich, dense granule protein of Toxoplasma gondii. Mol Biochem Parasitol 105:25–37
Cesbron-Delauw MF, Gendrin C, Travier L, Ruffiot P, Mercier C (2008) Apicomplexa in mammalian cells: trafficking to the parasitophorous vacuole. Traffic 9:657–664
Chen AL, Kim EW, Toh JY, Vashisht AA, Rashoff AQ, Van C, Huang AS, Moon AS, Bell HN, Bentolila LA, Wohlschlegel JA, Bradley PJ (2015) Novel components of the Toxoplasma inner membrane complex revealed by BioID. MBio. 6:e02357–e02314
Chen AL, Moon AS, Bell HN, Huang AS, Vashisht AA, Toh JY, Lin AH, Nadipuram SM, Kim EW, Choi CP, Wohlschlegel JA, Bradley PJ (2017) Novel insights into the composition and function of the Toxoplasma IMC sutures. Cell Microbiol 19:e12678
Del Carmen MG, Mondragon M, Gonzalez S, Mondragon R (2009) Induction and regulation of conoid extrusion in Toxoplasma gondii. Cell Microbiol 11:967–982
Donnelly SF, Pocklington MJ, Pallotta D, Orr E (1993) A proline-rich protein, verprolin, involved in cytoskeletal organization and cellular growth in the yeast Saccharomyces cerevisiae. Mol Microbiol 10:585–596
Endo T, Sethi KK, Piekarski G (1982) Toxoplasma gondii: calcium ionophore A23187-mediated exit of trophozoites from infected murine macrophages. Exp Parasitol 53:179–188
Fourniol F, Perderiset M, Houdusse A, Moores C (2013) Structural studies of the doublecortin family of MAPs. Methods Cell Biol 115:27–48
Freeman NL, Lila T, Mintzer KA, Chen Z, Pahk AJ, Ren R, Drubin DG, Field J (1996) A conserved proline-rich region of the Saccharomyces cerevisiae cyclase-associated protein binds SH3 domains and modulates cytoskeletal localization. Mol Cell Biol 16:548–556
Frixione E, Mondragon R, Meza I (1996) Kinematic analysis of Toxoplasma gondii motility. Cell Motil Cytoskeleton 34:152–163
Gilk SD, Gaskins E, Ward GE, Beckers CJ (2009) GAP45 phosphorylation controls assembly of the Toxoplasma myosin XIV complex. Eukaryot Cell 8:190–196
Gilk SD, Raviv Y, Hu K, Murray JM, Beckers CJ, Ward GE (2006) Identification of PhIL1, a novel cytoskeletal protein of the Toxoplasma gondii pellicle, through photosensitized labeling with 5-[125I]iodonaphthalene-1-azide. Eukaryot Cell 5:1622–1634
Gold DA, Kaplan AD, Lis A, Bett GC, Rosowski EE, Cirelli KM, Bougdour A, Sidik SM, Beck JR, Lourido S, Egea PF, Bradley PJ, Hakimi MA, Rasmusson RL, Saeij JP (2015) The Toxoplasma dense granule proteins GRA17 and GRA23 mediate the movement of small molecules between the host and the parasitophorous vacuole. Cell Host Microbe 17:642–652
Gomez de Leon CT, Diaz Martin RD, Mendoza Hernandez G, Gonzalez Pozos S, Ambrosio JR, Mondragon Flores R (2014) Proteomic characterization of the subpellicular cytoskeleton of Toxoplasma gondii tachyzoites. J Proteome 111:86–99
Gonzalez-Zamorano M, Mendoza-Hernandez G, Xolalpa W, Parada C, Vallecillo AJ, Bigi F, Espitia C (2009) Mycobacterium tuberculosis glycoproteomics based on ConA-lectin affinity capture of mannosylated proteins. J Proteome Res 8:721–733
Guerin A, El Hajj H, Penarete-Vargas D, Besteiro S, Lebrun M (2017) RON4L1 is a new member of the moving junction complex in Toxoplasma gondii. Sci Rep 7:17907
Hu K, Roos DS, Murray JM (2002) A novel polymer of tubulin forms the conoid of Toxoplasma gondii. J Cell Biol 156:1039–1050
Jean DC, Baas PW, Black MM (2012) A novel role for doublecortin and doublecortin-like kinase in regulating growth cone microtubules. Hum Mol Genet 21:5511–5527
Katris NJ, van Dooren GG, McMillan PJ, Hanssen E, Tilley L, Waller RF (2014) The apical complex provides a regulated gateway for secretion of invasion factors in Toxoplasma. PLoS Pathog 10:e1004074
Khater EI, Sinden RE, Dessens JT (2004) A malaria membrane skeletal protein is essential for normal morphogenesis, motility, and infectivity of sporozoites. J Cell Biol 167:425–432
Kim YR, Kim JS, Yun JS, Kim S, Kim SY, Jang K, Yang CS (2018) Toxoplasma gondii GRA8 induces ATP5A1-SIRT3-mediated mitochondrial metabolic resuscitation: a potential therapy for sepsis. Exp Mol Med 50:e464
Kozakov D, Hall DR, Xia B, Porter KA, Padhorny D, Yueh C, Beglov D, Vajda S (2017) The ClusPro web server for protein-protein docking. Nat Protoc 12:255–278
Lopez J, Bittame A, Massera C, Vasseur V, Effantin G, Valat A, Buaillon C, Allart S, Fox BA, Rommereim LM, Bzik DJ, Schoehn G, Weissenhorn W, Dubremetz JF, Gagnon J, Mercier C, Cesbron-Delauw MF, Blanchard N (2015) Intravacuolar membranes regulate CD8 T cell recognition of membrane-bound Toxoplasma gondii protective antigen. Cell Rep 13:2273–2286
Matsushima K, Tokuraku K, Hasan MR, Kotani S (2012) Microtubule-associated protein 4 binds to actin filaments and modulates their properties. J Biochem 151:99–108
Mercier C, Adjogble KD, Daubener W, Delauw MF (2005) Dense granules: are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites? Int J Parasitol 35:829–849
Mercier C, Cesbron-Delauw MF (2015) Toxoplasma secretory granules: one population or more? Trends Parasitol 31:60–71
Mondragon R, Frixione E (1996) Ca(2+)-dependence of conoid extrusion in Toxoplasma gondii tachyzoites. J Eukaryot Microbio 43:120–127
Morrissette N (2015) Targeting Toxoplasma tubules: tubulin, microtubules, and associated proteins in a human pathogen. Eukaryot Cell 14:2–12
Morrissette NS, Sibley LD (2002a) Cytoskeleton of apicomplexan parasites. Microbiol Mol Biol Rev 66:21–38 table of contents
Morrissette NS, Sibley LD (2002b) Disruption of microtubules uncouples budding and nuclear division in Toxoplasma gondii. J Cell Sci 115:1017–1025
Mueller C, Klages N, Jacot D, Santos JM, Cabrera A, Gilberger TW, Dubremetz JF, Soldati-Favre D (2013) The Toxoplasma protein ARO mediates the apical positioning of rhoptry organelles, a prerequisite for host cell invasion. Cell Host Microbe 13:289–301
Muniz-Hernandez S, Carmen MG, Mondragon M, Mercier C, Cesbron MF, Mondragon-Gonzalez SL, Gonzalez S, Mondragon R (2011) Contribution of the residual body in the spatial organization of Toxoplasma gondii tachyzoites within the parasitophorous vacuole. J Biomed Biotechnol 2011:473983
Nadano D, Nakayama J, Matsuzawa S, Sato TA, Matsuda T, Fukuda MN (2002) Human tastin, a proline-rich cytoplasmic protein, associates with the microtubular cytoskeleton. Biochem J 364:669–677
Nichols BA, Chiappino ML (1987) Cytoskeleton of Toxoplasma gondii. J Protozool 34:217–226
Nichols BA, Chiappino ML, O'Connor GR (1983) Secretion from the rhoptries of Toxoplasma gondii during host-cell invasion. J Ultrastruct Res 83:85–98
Orosz F (2011) Apicomplexan apicortins possess a long disordered N-terminal extension. Infect Genet Evol 11:1037–1044
Patron SA, Mondragon M, Gonzalez S, Ambrosio JR, Guerrero BA, Mondragon R (2005) Identification and purification of actin from the subpellicular network of Toxoplasma gondii tachyzoites. Int J Parasitol 35:883–894
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612. https://doi.org/10.1002/jcc.20084
Rommereim LM, Bellini V, Fox BA, Petre G, Rak C, Touquet B, Aldebert D, Dubremetz JF, Cesbron-Delauw MF, Mercier C, Bzik DJ (2016) Phenotypes associated with knockouts of eight dense granule gene loci (GRA2-9) in virulent Toxoplasma gondii. PLoS One 11:e0159306
Strunnikov AV (1998) SMC proteins and chromosome structure. Trends Cell Biol 8:454–459
Tonkin ML, Roques M, Lamarque MH, Pugniere M, Douguet D, Crawford J, Lebrun M, Boulanger MJ (2011) Host cell invasion by apicomplexan parasites: insights from the co-structure of AMA1 with a RON2 peptide. Science 333:463–467
Tran JQ, Li C, Chyan A, Chung L, Morrissette NS (2012) SPM1 stabilizes subpellicular microtubules in Toxoplasma gondii. Eukaryot Cell 11:206–216
Yang J, Zhang Y (2015) I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res 43:W174–W181
Acknowledgements
The authors are indebted to C.J. Beckers (Department of Cell and Developmental Biology, University of North Carolina, Chapel Hill, NC, USA); D. Soldati-Favre (Department of Microbiology and Molecular Medicine, CMU, Faculty of Medicine, University of Geneva, Switzerland); L.D. Sibley (Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO, USA); and M.F. Delauw (CNRS UMR 5525, Université Grenoble Alpes, France) for sharing reagents. RDDM, CTGL, and RMG were supported by the doctoral fellowships #324969, #324983, and #394378 from CONACyT-México, respectively. We thank Mónica Mondragón Castelán and Carlos J. Ramírez Flores for her technical support. Micrographs were obtained at the Electron Microscopy Unit (LANSE), CINVESTAV-IPN, México. Tandem Mass Spectrometry analysis was performed at the Proteomics, Genomics and Metabolomic Facility, LANSE-CINVESTAV-IPN, México.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Dana Mordue
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure 1
Titration of the rabbit serum anti-GRA8 used in immunoblot- and indirect immunofluorescence assays. A.20 μg per lane of tachyzoites whole cell extract were separated by SDS-PAGE, transferred to nitrocellulose, probed with the rabbit serum anti-GRA8 used at the dilutions 1/500, 1/1000 or 1/5000, and revealed with goat anti-rabbit IgG (H + L) coupled to peroxidase and enhanced chemiluminescence. B. Extracellular parasites were fixed, permeabilized and incubated with rabbit serum anti-GRA8 at the dilution 1/1000, followed by goat anti-rabbit IgG (H + L) coupled to TRITC. The parasite nucleus was revealed with DAPI. Scale bar = 5 μm (PDF 213 kb)
Supplementary Figure 2
TEM micrographs of the 21 serial sections from a RH WT tachyzoite (A) versus the 22 serial sections from a Δgra8 parasite (B), all used to count the dense granules present in the apical end versus the posterior end of each parasite. See the sections N° 8 in both series as examples of the quantified zones. Numbering of the serial sections is indicated in the upper right corner of each micrograph. The parasites, the serial sections of which were observed and photographed are indicated by white asterisks. The number of dense granules counted in the parasites here shown is indicated at the bottom of each micrograph composition. Scale bars = 2 μm. The same type of quantification was realized in about 10 parasites for each strain. (PDF 44930 kb)
Supplementary Figure 3
(PDF 17.6 mb)
Supplementary Figure 4
Structural organization of the subpellicular cytoskeleton of the Δgra4strain. TEM micrographs of a cytoskeleton isolated from tachyzoites of the Δgra4 strain at low magnification (A), at the apical end (B), at the level of the subpellicular microtubules and the network (C) and at the posterior end (D) respectively. Frames in A correspond to the respective magnifications in C-D. Scale bar = 0.3 μm. (PDF 1260 kb)
Rights and permissions
About this article
Cite this article
Díaz-Martín, R.D., Mercier, C., Gómez de León, C.T. et al. The dense granule protein 8 (GRA8) is a component of the sub-pellicular cytoskeleton in Toxoplasma gondii. Parasitol Res 118, 1899–1918 (2019). https://doi.org/10.1007/s00436-019-06298-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06298-7