Abstract
We describe a male patient (patient DGAP113) with a balanced translocation, 46,XY,t(1;3)(q31.3;q13.13), severe bilateral congenital cataracts, CNS abnormalities and mild developmental delay. Fluorescence in situ hybridization (FISH) and suppression PCR demonstrated that the chromosome 3 breakpoint lies ~515 kb upstream of the PVRL3 gene, while the chromosome 1 breakpoint lies ~50 kb upstream of the NEK7 gene. Despite the fact that NEK7 is closer to a translocation breakpoint than PVRL3, NEK7 transcript levels are unaltered in patient DGAP113 lymphoblastoid cells and Nek7-deficient mice exhibit no detectable ocular phenotype. In contrast, the expression of PVRL3, which encodes the cell adhesion protein Nectin 3, is significantly reduced in patient DGAP113 lymphoblastoid cells, likely due to a position effect caused by the chromosomal translocation. Nectin 3 is expressed in the mouse embryonic ciliary body and lens. Moreover, Pvrl3 knockout mice as well as a spontaneous mouse mutant ari (anterior retinal inversion), that maps to the Pvrl3 locus, exhibit lens and other ocular defects involving the ciliary body. Collectively, these data identify PVRL3 as a critical gene involved in a Nectin-mediated cell–cell adhesion mechanism in human ocular development.









Similar content being viewed by others
References
Alkuraya FS, Saadi I, Lund JJ, Turbe-Doan A, Morton CC, Maas RL (2006) SUMO1 haploinsufficiency leads to cleft lip and palate. Science 313(5794):1751
Azuma N, Hirakiyama A, Inoue T, Asaka A, Yamada M (2000) Mutations of a human homologue of the Drosophila eyes absent gene (EYA1) detected in patients with congenital cataracts and ocular anterior segment anomalies. Hum Mol Genet 9(3):363–366
Barron MJ, Brookes SJ, Draper CE, Garrod D, Kirkham J, Shore RC, Dixon MJ (2008) The cell adhesion molecule nectin-1 is critical for normal enamel formation in mice. Hum Mol Genet 17:3509–3520
Bassnett S, Beebe D (2001) Lens fiber differentiation. In: Robinson ML, Lovicuo F (eds) Development of the ocular lens, Chap 9, pp 214–244
Beebe DC, Vasiliev O, Guo J, Shui YB, Bassnett S (2001) Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Invest Ophthalmol Vis Sci 42(3):727–734
Bermejo E, Martinez-Frias ML (1998) Congenital eye malformations: clinical-epidemiological analysis of 1, 124, 654 consecutive births in Spain. Am J Med Genet 75(5):497–504
Bernacki SH, Stankovic AK, Williams LO, Beck JC, Herndon JE, Snow-Bailey K, Prior TW, Matteson KJ, Wasserman LM, Cole EC, Stenzel TT (2003) Establishment of stably EBV transformed cell lines from residual clinical blood samples for use in performance evaluation and quality assurance in molecular genetic testing. J Mol Diagn 5(4):227–230
Berry V, Francis P, Kaushal S, Moore A, Bhattacharya S (2000) Missense mutations in MIP underlie autosomal dominant ‘polymorphic’ and lamellar cataracts linked to 12q. Nat Genet 25(1):15–17
Bu L, Jin Y, Shi Y, Chu R, Ban A, Eiberg H, Andres L, Jiang H, Zheng G, Qian M, Cui B, Xia Y, Liu J, Hu L, Zhao G, Hayden MR, Kong X (2002) Mutant DNA-binding domain of HSF4 is associated with autosomal dominant lamellar and Marner cataract. Nat Genet 31(3):276–278
Chauhan BK, Disanza A, Choi SY, Faber SC, Lou M, Beggs HE, Scita G, Zheng Y, Lang RA (2009) Cdc42- and IRSp53-dependent contractile filopodia tether presumptive lens and retina to coordinate epithelial invagination. Development 136(21):3657–3667
Chen J, Ma Z, Jiao X, Fariss R, Kantorow WL, Kantorow M, Pras E, Frydman M, Pras E, Riazuddin S, Riazuddin SA, Hejtmancik JF (2011) Mutations in FYCO1 cause autosomal-recessive congenital cataracts. Am J Hum Genet 88(6):827–838
Choe SE, Boutros M, Michelson AM, Church GM, Halfon MS (2005) Preferred analysis methods for Affymetrix GeneChips revealed by a wholly defined control dataset. Genome Biol 6(2):R16
Conley YP, Erturk D, Keverline A, Mah TS, Keravala A, Barnes LR, Bruchis A, Hess JF, FitzGerald PG, Weeks DE, Ferrell RE, Gorin MB (2000) A juvenile-onset, progressive cataract locus on chromosome 3q21–q22 is associated with a missense mutation in the beaded filament structural protein-2. Am J Hum Genet 66(4):1426–1431
Davies AF, Mirza G, Flinter F, Ragoussis J (1999) An interstitial deletion of 6p24–p25 proximal to the FKHL7 locus and including AP-2alpha that affects anterior eye chamber development. J Med Genet 36(9):708–710
Donner AL, Lachke SA, Maas RL (2006) Lens induction in vertebrates: variations on a conserved theme of signaling events. Semin Cell Dev Biol 17(6):676–685
Fabre S, Reymond N, Cocchi F, Menotti L, Dubreuil P, Campadelli-Fiume G, Lopez M (2002) Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C-C’-C”-D beta-strands of the nectin1 V domain. J Biol Chem 277(30):27006–27013
Feige E, Motro B (2002) The related murine kinases, Nek6 and Nek7, display distinct pattern of expression. Mech Dev 110(1–2):219–223
Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL (1994) PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet 7(4):463–471
Graw J (2009) Mouse models of cataract. J Genet 88(4):469–486
Heintzman ND, Stuart RK, Hon G, Fu Y et al (2007) Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39(3):311–318
Hejtmancik JF (2008) Congenital cataracts and their molecular genetics. Semin Cell Dev Biol 19(2):134–149
Higgins AW, Alkuraya FS, Bosco AF, Brown KK, Bruns GA, Donovan DJ, Eisenman R, Fan Y, Farra CG, Ferguson HL, Gusella JF, Harris DJ, Herrick SR, Kelly C, Kim HG, Kishikawa S, Korf BR, Kulkarni S, Lally E, Leach NT, Lemyre E, Lewis J, Ligon AH, Lu W, Maas RL, MacDonald ME, Moore SD, Peters RE, Quade BJ, Quintero-Rivera F, Saadi I, Shen Y, Shendure J, Williamson RE, Morton CC (2008) Characterization of apparently balanced chromosomal rearrangements from the developmental genome anatomy project. Am J Hum Genet 82(3):712–722
Hon G, Ren B, Wang W (2008) ChromaSig: a probabilistic approach to finding common chromatin signatures in the human genome. PLoS Comput Biol 4(10):e1000201
Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Morimoto K, Inoue E, Ohtsuka T, Miyoshi J, Takai Y (2005) Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development 132(7):1525–1537
Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Takai Y (2006) Role of cell adhesion molecule nectin-3 in spermatid development. Genes Cells 11(9):1125–1132
Jamieson RV, Perveen R, Kerr B, Carette M, Yardley J, Heon E, Wirth MG, van Heyningen V, Donnai D, Munier F, Black GC (2002) Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum Mol Genet 11(1):33–42
Jensen S, Goldschmidt E (1971) Genetic counselling in sporadic cases of congenital cataract. Acta Ophthalmol (Copenh) 49(4):572–576
Kandli M, Feige E, Chen A, Kilfin G, Motro B (2000) Isolation and characterization of two evolutionarily conserved murine kinases (Nek6 and nek7) related to the fungal mitotic regulator, NIMA. Genomics 68(2):187–196
Kimura M, Okano Y (2001) Identification and assignment of the human NIMA-related protein kinase 7 gene (NEK7) to human chromosome 1q31.3. Cytogenet Cell Genet 94(1–2):33–38
Kleinjan DJ, van Heyningen V (1998) Position effect in human genetic disease. Hum Mol Genet 7(10):1611–1618
Kleinjan DJ, van Heyningen V (2005) Long-range control of gene expression: emerging mechanisms and disruption in disease. Am J Hum Genet 76(1):8–32
Kuszak JR, Novak LA, Brown HG (1995) An ultrastructural analysis of the epithelial-fiber interface (EFI) in primate lenses. Exp Eye Res 61(5):579–597
Lachke SA, Maas RL (2010) Building the developmental oculome: systems biology in vertebrate eye development and disease. Wiley Inter Rev: Sys Biol Med 2(3):305–323
Lachke SA, Alkuraya FS, Kneeland S, Ohn T, Aboukhalil A, Howell G, Saadi I, Cavallesco R, Yingzi Y, Tsai AC, Nair SK, Cosma MI, Smith RS, Hodges E, AlFadhli SM, Al-Hajeri A, Shamseddin H, Behbehani A, Hannon G, Bulyk ML, Drack AV, Anderson PJ, John SW, Maas RL (2011) Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science 331(6024):1571–1576
Lauderdale JD, Wilensky JS, Oliver ER, Walton DS, Glaser T (2000) 3′ deletions cause aniridia by preventing PAX6 gene expression. Proc Natl Acad Sci USA 97(25):13755–13759
Le TT, Conley KW, Brown NL (2009) Jagged 1 is necessary for normal mouse lens formation. Dev Biol 328(1):118–126
Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, Shiels A, Bhattacharya S (1999) Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet 64(5):1357–1364
Mizoguchi A, Nakanishi H, Kimura K, Matsubara K, Ozaki-Kuroda K, Katata T, Honda T, Kiyohara Y, Heo K, Higashi M, Tsutsumi T, Sonoda S, Ide C, Takai Y (2002) Nectin: an adhesion molecule involved in formation of synapses. J Cell Biol 156(3):555–565
Nguyen MM, Rivera C, Griep AE (2005) Localization of PDZ domain containing proteins discs large-1 and scribble in the mouse eye. Mol Vis 11:1183–1199
Nielsen PA, Baruch A, Shestopalov VI, Giepmans BN, Dunia I, Benedetti EL, Kumar NM (2003) Lens connexins alpha3Cx46 and alpha8Cx50 interact with zonula occludens protein-1 (ZO-1). Mol Biol Cell 14(6):2470–2481
O’Connell MJ, Krien MJ, Hunter T (2003) Never say never. The NIMA-related protein kinases in mitotic control. Trends Cell Biol 13(5):221–228
Ogita H, Takai Y (2008) Cross-talk among integrin, cadherin, and growth factor receptor: roles of nectin and nectin-like molecule. Int Rev Cytol 265:1–54
Ogita H, Rikitake Y, Miyoshi J, Takai Y (2010) Cell adhesion molecules nectins and associating proteins: Implications for physiology and pathology. Proc Jpn Acad Ser B Phys Biol Sci 86(6):621–629
Okabe N, Ozaki-Kuroda K, Nakanishi H, Shimizu K, Takai Y (2004) Expression patterns of nectins and afadin during epithelial remodeling in the mouse embryo. Dev Dyn 230(1):174–186
Pontoriero GF, Smith AN, Miller LA, Radice GL, West-Mays JA, Lang RA (2009) Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev Biol 326(2):403–417
Pras E, Levy-Nissenbaum E, Bakhan T, Lahat H, Assia E, Geffen-Carmi N, Frydman M, Goldman B, Pras E (2002) A missense mutation in the LIM2 gene is associated with autosomal recessive presenile cataract in an inbred Iraqi Jewish family. Am J Hum Genet 70(5):1363–1367
Prosser J, van Heyningen V (1998) PAX6 mutations reviewed. Hum Mutat 11(2):93–108
Ramachandran RD, Perumalsamy V, Hejtmancik JF (2007) Autosomal recessive juvenile onset cataract associated with mutation in BFSP1. Hum Genet 121(3–4):475–482
Reymond N, Borg J-P, Lecocq E, Adelaide J, Campadelli-Fiume G, Dubreuil P, Lopez M (2000) Human nectin3/PRR3: a novel member of the PVR/PRR/nectin family that interacts with afadin. Gene 255(2):347–355
Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA (1995) Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development 121(2):505–514
Roessler E, Ward DE, Gaudenz K, Belloni E, Scherer SW, Donnai D, Siegel-Bartelt J, Tsui LC, Muenke M (1997) Cytogenetic rearrangements involving the loss of the Sonic Hedgehog gene at 7q36 cause holoprosencephaly. Hum Genet 100(2):172–181
Salem H, Rachmin I, Yissachar N, Cohen S, Amiel A, Haffner R, Lavi L, Motro B (2010) Nek7 kinase targeting leads to early mortality, cytokinesis disturbance and polyploidy. Oncogene 29(28):4046–4057
Sandilands A, Prescott AR, Wegener A, Zoltoski RK, Hutcheson AM, Masaki S, Kuszak JR, Quinlan RA (2003) Knockout of the intermediate filament protein CP49 destabilises the lens fibre cell cytoskeleton and decreases lens optical quality, but does not induce cataract. Exp Eye Res 76(3):385–391
Saravanamuthu SS, Gao CY, Zelenka PS (2009) Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation. Dev Biol 332(1):166–176
Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y (2000) Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell–cell adhesion activities. J Biol Chem 275(14):10291–10299
Seidman JG, Seidman C (2002) Transcription factor haploinsufficiency: when half a loaf is not enough. J Clin Invest 109(4):451–455
Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WL, Reiter RS, Funkhauser C, Daack-Hirsch S, Murray JC (1998) A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat Genet 19(2):167–170
Semina EV, Brownell I, Mintz-Hittner HA, Murray JC, Jamrich M (2001) Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum Mol Genet 10(3):231–236
Shiels A, Hejtmancik JF (2007) Genetic origins of cataract. Arch Ophthalmol 125(2):165–173
Shiels A, Bennett TM, Hejtmancik JF (2010) Cat-Map: putting cataract on the map. Mol Vis 16:2007–2015
Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S (1998) A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet 62(3):526–532
Shiels A, Bennett TM, Knopf HL, Maraini G, Li A, Jiao X, Hejtmancik JF (2008) The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol Vis 14:2042–2055
Siebert PD, Chenchik A, Kellogg DE, Lukyanov KA, Lukyanov SA (1995) An improved PCR method for walking in uncloned genomic DNA. Nuc Acids Res 23(6):1087–1088
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98(3):503–517
Takai Y, Ikeda W, Ogita H, Rikitake Y (2008a) The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol 24:309–342
Takai Y, Miyoshi J, Ikeda W, Ogita H (2008b) Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol 9(8):603–615
Takekuni K, Ikeda W, Fujito T, Morimoto K, Takeuchi M, Monden M, Takai Y (2003) Direct binding of cell polarity protein PAR-3 to cell–cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem 278(8):5497–5500
Wallis DE, Roessler E, Hehr U, Nanni L, Wiltshire T, Richieri-Costa A, Gillessen-Kaesbach G, Zackai EH, Rommens J, Muenke M (1999) Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly. Nat Genet 22(2):196–198
White TW, Goodenough DA, Paul DL (1998) Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol 143:815–825
Xu L, Overbeek PA, Reneker LA (2002) Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp Eye Res 74(6):753–760
Acknowledgments
We thank the subject and his relatives for participating in the Developmental Genome Anatomy Project and Heather Ferguson, Chantal Kelly and Shahrin Ahsan for assistance as study coordinators. This work was supported by NIH grants 5R01EY10123-15 (RLM), 5R01HD060050-02 (RLM), 5P01GM061354-07 (CCM, RLM, JFG, BJQ), R01EY021505 (SAL), R01EY12995 (MLR), and T35-EY07151 (QTD). All experiments comply with the current laws of the countries (US, Israel and Japan) in which they were performed.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. A. Lachke and A. W. Higgins contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental data include five figures and one table and can be found with this article online.
Supplemental Fig. 1. Nek7 hypomorphic mutant mice do not exhibit lens defects. (A) Reverse Transcriptase (RT)-PCR analysis of E12.5 mouse embryonic ocular tissue from wild type (+/+), heterozygous (±), and homozygous (-/-) littermates for the Sanger Nek7 gene trap allele demonstrates that the gene trap generates a hypomorphic Nek7 allele. (B) Quantitative RT-PCR analysis of E12.5 mouse embryonic ocular tissue from wild type (+/+), heterozygous (±), and homozygous (-/-) littermates for the Sanger Nek7 gene trap allele confirms that the gene trap generates a hypomorphic Nek7 allele. (C-E) Staining for β-galactosidase activity in (C) wild type (WT), (D) heterozygous (Nek7 ±), and (E) homozygous (Nek7-/-) for the Sanger Nek7 gene trap allele that contains a knock-in LacZ transgene allele. (F–H) Staining for β-galactosidase activity in mouse Nek7-/- embryos homozygous for the Sanger Nek7 gene trap allele at (F) E10.5, (G) E11.5, and (H) E12.5 demonstrates increased LacZ transgene expression in the eye as development occurs. Lens abnormality or cataract was not detected in Nek7-/- hypomorphic mice at embryonic (above) and adult stages (data not shown), or in Nek7 nullizygotes.
Supplemental Fig. 2. Nek7 null mutant mice do not exhibit lens defects at post-natal data 5 (P5). (A) H&E stained section of P5 wild type eye. (B) H&E stained section of P5 Nek7-/- mutant eye demonstrates the absence of an obvious lens or ocular defect. Abbr.: Co, cornea; Le, lens; Rt, retina.
Supplemental Fig. 3. Nectin 3 is expressed in the developing lens and prospective ciliary epithelia. (A) Immunofluorescence microscopy (IFM) of E14.5 mouse eye demonstrates Nectin 3 expression (red) in the AEL. (A’) High magnification of boxed region in (A). Polarized staining of Nectin 3 in the AEL is indicated by arrow. (B) IFM of E13.5 mouse embryos demonstrates Nectin 3 expression in the presumptive ciliary epithelium. PE, Pigment epithelium. (B’) High magnification of boxed region in (B). Nectin-3 staining indicated by arrow. (C) IFM of E14.5 mouse embryos demonstrates Nectin 3 expression in the presumptive ciliary epithelium. NPE, Non-pigmented epithelium. (C’) High magnification of boxed region in (C). Polarized staining of Nectin 3 in NPE is indicated by arrow. (D) IFM of E18.5 mouse embryos at E18.5 demonstrates expression of Nectin 3 in the presumptive ciliary epithelium. (D’) High magnification of boxed region in (D). Polarized Nectin 3 expression in NPE is indicated by arrow. Other abbr.: AEL, Anterior epithelium of the lens; FC, Fiber cells. Scale bars: (A) 15 μm, (A’) 8 μm, (B) 16 μm, (B’) 6 μm, (C) 10 μm, (C’) 7 μm, (D) 15 μm, (D’) 10 μm.
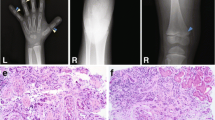
Supplemental Fig. 4. The ari mutation maps to the Pvrl3 locus and affects Pvrl3 expression in the prospective ciliary epithelia. (A) Genetic intervals between ari and the closest polymorphic markers were determined by examining the phenotype in recombinant backcross animals out of a total of 656 backcross progeny. Mice used to draw the conclusion that ari is located between D16Mit126 and D16Mit61 are summarized in Supplementary Table 1. (B) Physical distance encompassing the ari genetic interval according to mouse genome databases. The physical interval where ari lies is approximately 2.3 Mb. The Pvrl3 gene is approximately 109 kb. The distance between D16Mit126 and the 5’ end of Pvrl3 is 415 kb, and the distance between D16Mit61 and the 3’ end of Pvrl3 is 1.8 Mb. (C) Hematoxylin-stained section demonstrates morphology and (D) section in situ analysis on ocular region of wild type littermate mouse demonstrates normal Pvrl3 expression (arrow) in E15.5 presumptive ciliary epithelia. (C’) Hematoxylin-stained section demonstrates morphology and (D’) section in situ analysis on ocular region of an E15.5 ari mouse mutant demonstrates background levels of Pvrl3 expression (asterisk) in the presumptive ciliary epithelium. The retina region is outlined by dashed line. Abbr.: npe, non pigment epithelium; pe, pigment epithelium; r, retina.
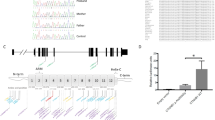
Supplemental Fig. 5. Chromosome 3 breakpoint separates five putative long-distance regulatory elements upstream of PVRL3. (A) Schematic representation of human chromosome 3 with red box showing region highlighted in (B). (B) Chromatin Immunoprecipitation followed by DNA sequencing (ChIP-seq) analysis in eight human cell lines identifies several loci (green boxes) that display Histone 3 lysine 4 mono-methylation (H3K4Me1) and histone 3 lysine 4 acetylation (H3K27Ac) of the chromosome 3 genomic region upstream of PVRL3 and the chromosomal 3 breakpoint (dashed red line) which is separated from the PVRL3 transcription unit by patient DGAP113’s translocation. However, no significant signal for tri-methylated Histone 3 lysine 4 (H3KMe3) in this genomic region is observed, also compatible with the presence of long-range regulatory elements. In constrast, significant tri-methylated Histone 3 lysine 4 (H3KMe3) signals (blue boxes) are observed only near the PVRL3 and DPPA4 transcription start sites, indicative of promoter-driven regulatory elements.
Supplemental Table 1 Summary of the genetic backcross analysis performed to map the ari mutation. Abbr.: C: Cataract; H: Heterozygous for the microsatellite polymorphism (FVB/N and C57BL/6); N: Normal eye; F: Homozygous for the FVB/N polymorphism. Microsatellite polymorphisms are arranged left to right in a centromeric to telomeric orientation on mouse chromosome 16. Note: Mice used to draw the conclusion that ari is located between D16Mit126 and D16Mit61 are summarized in red letters. Mouse (“Animal”) 598 demonstrates that the mutation must be telomeric to D16Mit126. Mouse 694 demonstrates that the mutation must be telomeric to D16Mit59. Mouse 143 demonstrates that the mutation must be telomeric to D16Mit84. Mouse 272 demonstrates that the mutation must be centromeric to D16Mit61. Mouse 611 demonstrates that the mutation must be telomeric to D16Mit84. Mouse 695 demonstrates that the mutation must be telomeric to D16Mit126.
Rights and permissions
About this article
Cite this article
Lachke, S.A., Higgins, A.W., Inagaki, M. et al. The cell adhesion gene PVRL3 is associated with congenital ocular defects. Hum Genet 131, 235–250 (2012). https://doi.org/10.1007/s00439-011-1064-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-011-1064-z