Abstract
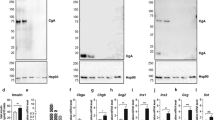
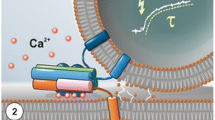
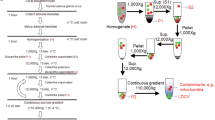
Neurons and certain kinds of endocrine cells, such as adrenal chromaffin cells, have large dense-core vesicles (LDCVs) and synaptic vesicles or synaptic-like microvesicles (SLMVs). These secretory vesicles exhibit differences in Ca2+ sensitivity and contain diverse signaling substances. The present work was undertaken to identify the synaptotagmin (Syt) isoforms present in secretory vesicles. Fractionation analysis of lysates of the bovine adrenal medulla and immunocytochemistry in rat chromaffin cells indicated that Syt 1 was localized in LDCVs and SLMVs, whereas Syt 7 was the predominant isoform present in LDCVs. In contrast to PC12 cells and the pancreatic β cell line INS-1, Syt 9 was not immunodetected in LDCVs in rat chromaffin cells. Double-staining revealed that Syt 9-like immunoreactivity was nearly identical with fluorescent thapsigargin binding, suggesting the presence of Syt 9 in the endoplasmic reticulum (ER).The exogenous expression of Syt 1-GFP in INS-1 cells, which had a negligible level of endogenous Syt 1, resulted in an increase in the amount of Syt 9 in the ER, suggesting that Syt 9 competes with Syt 1 for trafficking from the ER to the Golgi complex. We conclude that LDCVs mainly contain Syt 7, whereas SLMVs contain Syt 1, but not Syt 7, in rat and bovine chromaffin cells.







Similar content being viewed by others
References
Ahras M, Otto GP, Tooze SA (2006) Synaptotagmin IV is necessary for the maturation of secretory granules in PC12 cells. J Cell Biol 173:241–251
Chapman ER (2008) How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem 77:615–641
Courel M, Rodemer C, Nguyen ST, Pance A, Jackson AP, O’connor DT, Taupenot L (2006) Secretory granule biogenesis in sympathoadrenal cells: identification of a granulogenic determinant in the secretory prohormone chromogranin A. J Biol Chem 281:38038–38051
Craxton M (2007) Evolutionary genomics of plant genes encoding N-terminal-TM-C2 domain proteins and the similar FAM62 genes and synaptotagmin genes of metazoans. BMC Genomics 8:259
Fukuda M (2006) The role of synaptotagmin and synaptotagmin-like protein (Slp) in regulated exocytosis. In: Regazzi R (ed) Molecular mechanisms of exocytosis. Landes Bioscience, Austin, pp 42–61
Fukuda M, Mikoshiba K (2000) Calcium-dependent and -independent hetero-oligomerization in the synaptotagmin family. J Biochem 128:637–645
Fukuda M, Kowalchyk JA, Zhang X, Martin TF, Mikoshiba K (2002) Synaptotagmin IX regulates Ca2+-dependent secretion in PC12 cells. J Biol Chem 277:4601–4604
Fukuda M, Kanno E, Ogata Y, Saegusa C, Kim T, Peng Loh Y, Yamamoto A (2003) Nerve growth factor-dependent sorting of synaptotagmin IV protein to mature dense-core vesicles that undergo calcium-dependent exocytosis in PC12 cells. J Biol Chem 278:3220–3226
Fukuda M, Kanno E, Satoh M, Saegusa C, Yamamoto A (2004) Synaptotagmin VII is targeted to dense-core vesicles and regulates their Ca2+-dependent exocytosis in PC12 cells. J Biol Chem 279:52677–52684
Gauthier BR, Duhamel DL, Iezzi M, Theander S, Saltel F, Fukuda M, Wehrle-Haller B, Wollheim CB (2008) Synaptotagmin VII splice variants α, β, and δ are expressed in pancreatic β-cells and regulate insulin exocytosis. FASEB J 22:194–206
Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC (1994) Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79:717–727
Harada K, Matsuoka H, Nakamura J, Fukuda M, Inoue M (2010) Storage of GABA in chromaffin granules and not in synaptic-like microvesicles in rat adrenal medullary cells. J Neurochem 114:617–626
Hudson AW, Birnbaum MJ (1995) Identification of a nonneuronal isoform of synaptotagmin. Proc Natl Acad Sci USA 92:5895–5899
Hui E, Bai J, Wang P, Sugimori M, Llinas RR, Chapman ER (2005) Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis. Proc Natl Acad Sci USA 102:5210–5214
Ibata K, Hashikawa T, Tsuboi T, Terakawa S, Liang F, Mizutani A, Fukuda M, Mikoshiba K (2002) Non-polarized distribution of synaptotagmin IV in neurons: evidence that synaptotagmin IV is not a synaptic vesicle protein. Neurosci Res 43:401–406
Iezzi M, Kouri G, Fukuda M, Wollheim CB (2004) Synaptotagmin V and IX isoforms control Ca2+-dependent insulin exocytosis. J Cell Sci 117:3119–3127
Inoue M, Fujishiro N, Ogawa MM, Sakamoto Y, Imanaga I, Shioda S (2000) Pituitary adenylate cyclase-activating polypeptide may function as a neuromodulator in guinea-pig adrenal medulla. J Physiol (Lond) 528:473–487
Inoue M, Sakamoto Y, Fujishiro N, Imanaga I, Ozaki S, Prestwich GD, Warashina A (2003) Homogeneous Ca2+ stores in rat adrenal chromaffin cells. Cell Calcium 33:19–26
Inoue M, Harada K, Matsuoka H, Warashina A (2010) Paracrine role of GABA in adrenal chromaffin cells. Cell Mol Neurobiol 586:4825–4842
Karhunen T, Vilim FS, Alexeeva V, Weiss KR, Church PJ (2001) Targeting of peptidergic vesicles in cotransmitting terminals. J Neurosci 21:RC127
Kasai H (1999) Comparative biology of Ca2+-dependent exocytosis: implications of kinetic diversity for secretory function. Trends Neurosci 22:88–93
Kim T, Gondré-Lewis MC, Arnaoutova I, Loh YP (2006) Dense-core secretory granule biogenesis. Physiology 21:124–133
Lynch KL, Martin TFJ (2007) Synaptotagmins I and IX function redundantly in regulated exocytosis but not endocytosis in PC12 cells. J Cell Sci 120:617–627
Matsuoka H, Harada K, Endo Y, Warashina A, Doi Y, Nakamura J, Inoue M (2008) Molecular mechanisms supporting a paracrine role of GABA in rat adrenal medullary cells. J Physiol (Lond) 586:4825–4842
Matsuoka H, Harada K, Ikeda T, Uetsuki K, Sata T, Warashina A, Inoue M (2009) Ca2+ pathway involved in the refilling of store sites in rat adrenal medullary cells. Am J Physiol Cell Physiol 296:C889–C899
Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G (1995) Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol 131:1715–1726
Nasu-Nishimura Y, Hurtado D, Braud S, Tang TT, Isaac JT, Roche KW (2006) Identification of an endoplasmic reticulum-retention motif in an intracellular loop of the kainate receptor subunit KA2. J Neurosci 26:7014–7021
Navone F, Jahn R, Di Gioia G, Stukenbrok H, Greengard P, De Camilli P (1986) Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol 103:2511–2527
Reetz A, Solimena M, Matteoli M, Foll F, Takei K, De Camilli P (1991) GABA and pancreatic β-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J 10:1275–1284
Saegusa C, Fukuda M, Mikoshiba K (2002) Synaptotagmin V is targeted to dense-core vesicles that undergo calcium-dependent exocytosis in PC12 cells. J Biol Chem 277:24499–24505
Schonn JS, Maximov A, Lao Y, Südhof TC, Sørensen JB (2008) Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc Natl Acad Sci USA 105:3998–4003
Sørensen JB (2004) Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles. Pflugers Arch 448:347–362
Strasser JE, Arribas M, Blagoveshchenskaya AD, Cutler DF (1999) Secretagogue-triggered transfer of membrane proteins from neuroendocrine secretory granules to synaptic-like microvesicles. Mol Biol Cell 10:2619–2630
Südhof TC (2004) The synaptic vesicle cycle. Annu Rev Neurosci 27:509–547
Sugita S, Han W, Butz S, Liu X, Fernández-Chacón R, Lao Y, Südhof TC (2001) Synaptotagmin VII as a plasma membrane Ca2+ sensor in exocytosis. Neuron 30:459–473
Tandon A, Bannykh S, Kowalchyk JA, Banerjee A, Martin TF, Balch WE (1998) Differential regulation of exocytosis by calcium and CAPS in semi-intact synaptosomes. Neuron 21:147–154
Thomas-Reetz AC, De Camilli P (1994) A role for synaptic vesicles in non-neuronal cells: clues from pancreatic β cells and from chromaffin cells. FASEB J 8:209–216
Tooze SA, Flatmark T, Tooze J, Huttner WB (1991) Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol 115:1491–1503
Tucker WC, Edwardson JM, Bai J, Kim HJ, Martin TF, Chapman ER (2003) Identification of synaptotagmin effectors via acute inhibition of secretion from cracked PC12 cells. J Cell Biol 162:199–209
Vician L, Lim IK, Ferguson G, Tocco G, Baudry M, Herschman HR (1995) Synaptotagmin IV is an immediate early gene induced by depolarization in PC12 cells and in brain. Proc Natl Acad Sci USA 92:2164–2168
Walch-Solimena C, Takei K, Marek KL, Midyett K, Südhof TC, De Camilli P, Jahn R (1993) Synaptotagmin: a membrane constituent of neuropeptide-containing large dense-core vesicles. J Neurosci 13:3895–3903
Wang C-T, Lu J-C, Bai J, Chang PY, Martin TFJ, Chapman ER, Jackson MB (2003) Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature 424:943–947
Wang P, Chicka MC, Bhalla A, Richards DA, Chapman ER (2005) Synaptotagmin VII is targeted to secretory organelles in PC12 cells, where it functions as a high-affinity calcium sensor. Mol Cell Biol 25:8693–8702
Wang L-Y, Neher E, Taschenberger H (2008) Synaptic vesicles in mature calyx of Held synapses sense higher nanodomain calcium concentrations during action potential-evoked glutamate release. J Neurosci 28:14450–14458
Winkler H, Westhead E (1980) The molecular organization of adrenal chromaffin granules. Neuroscience 5:1803–1823
Wit H de, Lichtenstein Y, Geuze HJ, Kelly RB, Sluijs P van der, Klumperman J (1999) Synaptic vesicles from by budding from tubular extensions of sorting endosomes in PC12 cells. Mol Biol Cell 10:4163–4176
Xu J, Mashimo T, Südhof TC (2007) Synaptotagmin-1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron 54:567–581
Zhang X, Kim-Miller MJ, Fukuda M, Kowalchyk JA, Martin TFJ (2002) Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron 34:99–611
Acknowledgments
We thank I. Niki (Oita University, Oita, Japan) and C. B. Wollheim (University Medical Center, Geneva, Switzerland) for providing INS-1 cells. Our thanks are also due to M. Courel (University of California, San Diego, USA) for the plasmid pCMV-CgA-EGFP and to H.-H. Gerdes (University of Bergen, Bergen, Norway) for the plasmid pcDNA3-hCgB-EGFP. We are grateful to T. Hatama for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by MEXT KAKENHI (21026029 to M.I.) and JSPS KAKENHIs (21500360 to M.I. and 22790222 to H.M.).
Rights and permissions
About this article
Cite this article
Matsuoka, H., Harada, K., Nakamura, J. et al. Differential distribution of synaptotagmin-1, -4, -7, and -9 in rat adrenal chromaffin cells. Cell Tissue Res 344, 41–50 (2011). https://doi.org/10.1007/s00441-011-1131-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1131-8